Manvir Singh & Luke Glowacki
Human social organization during the Late Pleistocene
Beyond the nomadic-egalitarian model
March 2021
Limitations of using extant foragers as models of Late Pleistocene societies
Other interactions with states and agricultural societies
Mischaracterizations of recent foragers
The importance of considering sedentary and non-egalitarian foragers
Sedentary foragers are not anomalous
Humans have exploited aquatic resources deep into the Pleistocene
The evidence for sedentary hunter-gatherers during the Pleistocene
A new model of Late Pleistocene lifeways
Implications for the evolution of human behavior
Dominance and other status-seeking behaviors
Group identity and minimal group affiliation
Gene-culture coevolution and human social psychology
Manvir Singh1*, Luke Glowacki2
1Institute for Advanced Study in Toulouse
2Department of Anthropology, Pennsylvania State University
*Corresponding author: manvir.singh@iast.fr
Abstract
Many researchers assume that until 10-12,000 years ago, humans lived in small, mobile, relatively egalitarian bands composed mostly of kin. This “nomadic-egalitarian model” informs evolutionary explanations of behavior and our understanding of how contemporary societies differ from those of our evolutionary past. Here, we synthesize research challenging this model and propose an alternative, the diverse histories model, to replace it. We outline the limitations of using recent foragers as models of Late Pleistocene societies and the considerable social variation among foragers commonly considered small-scale, mobile, and egalitarian. We review ethnographic and archaeological findings covering 34 world regions showing that non-agricultural peoples often live in groups that are more sedentary, unequal, large, politically stratified, and capable of large-scale cooperation and resource management than is normally assumed. These characteristics are not restricted to extant Holocene hunter-gatherers but, as suggested by archaeological findings from 27 Middle Stone Age sites, likely characterized societies throughout the Late Pleistocene (until c. 130 ka), if not earlier. These findings have implications for how we understand human psychological adaptations and the broad trajectory of human history.
What did human societies look like before the Neolithic Revolution some 10-12,000 years ago? Through detailed field studies of mobile hunter-gatherers such as the Ju/’hoansi, Ache, and Hadza1–4 and systematic comparisons of (mostly mobile) hunter-gatherers5–8, evolutionary anthropologists have developed a model of pre-Holocene lifeways—a model that represents the conditions under which our species evolved and from which modern societies developed. We refer to this as the nomadic-egalitarian model and characterize it as follows (for recent formulations by anthropologists, see work by Fry9,10, Lee11, and Boehm12):
For tens of thousands of years before the Holocene, and possibly much earlier (e.g., ≥ 200 ka),
People lived in small bands of up to several dozen individuals. Any focal individual was likely related to most fellow band members, either through blood or marriage. Bands were embedded with ethnolinguistic groups, which comprised hundreds or, in rare cases, a couple thousand individuals.
Bands were mobile and fluid, and people stored very little, relying instead on sharing to insure against risk. As a result, people had few material possessions, and notions of property were weak.
Social relationships were egalitarian, at least among individuals of similar age and sex. Egalitarianism was maintained both by minimal differences in wealth and by leveling mechanisms such as gossip, teasing, and the threat of coordinated violence.
Cooperation was small-scale, occurring mostly among fellow band members.
Agriculture, comprising cultivation and the management of animal populations, was absent.
This model dominates evolutionary analyses of behavior, both as researchers consider how particular behaviors may have been adaptive in mobile, egalitarian, small-scale settings and as they study those behaviors in contemporary populations to make inferences about the past. Indeed, the nomadic, egalitarian model has become an important lens through which scholars, including us, have studied behaviors as diverse as aggression13,14, childcare15, cooperation16,17, cumulative culture18–20, leadership21,22, morality12, religion23, the sexual division of labor24, social emotion15, storytelling25,26, violence11,27, and warfare28,29.
Because it posits that “historically nomadic foragers (HNF), small in scale, mobile, and egalitarian, reflect most closely the characteristics of ancient foragers”11, the nomadic-egalitarian model casts many features of contemporary societies as historically novel and in need of explanation. If humans evolved in contexts where they cooperated with, at most, dozens of individuals, then large-scale cooperation becomes a puzzle30,31. If humans evolved in contexts where status-seeking or domineering behavior was constantly suppressed16,32, then the emergence of stratification and inherited inequality becomes a puzzle33,34. If, during 95% of our species’ history, we lacked property35–37 or cultivation38,39 or large-scale political consolidation and decision-making40, then the emergence and ubiquity of those phenomena demands explanation. Depending on how one interprets ethnographic reports of mobile foragers, either the modern capacity for peace28 or the predilection for war10,29 are also historically peculiar and, as a result, demand special explanation.
Although the nomadic-egalitarian model has long attracted skepticism41, researchers in recent years have expressed a growing dissatisfaction with features of the model42–46. Here, we synthesize this and other literature and propose a new model of pre-Holocene lifeways, referred to here as the diverse histories model. As early as the penultimate interglacial (c. 130 ka)—and possibly much earlier—humans lived in societies that varied considerably in their social organization. Some humans lived in large, sedentary, dense communities. Some lived in stratified societies with inherited status. Some engaged in cooperative projects with hundreds, even thousands, of people. Some cultivated plants and managed animal populations and may have even domesticated species. As a result, human psychology is adapted not just to dwell in small, relatively egalitarian bands, but to flexibly traverse a broader range of social environments. This new model of human psychological evolution helps explain many reliably developing behaviors that are otherwise difficult to explain if most of our recent evolution was spent in small, mobile, egalitarian bands.
The rest of the paper is structured as follows: We first outline weaknesses of the empirical foundation of the nomadic-egalitarian model. We review limitations of using extant foragers as the primary models of Late Pleistocene and highlight the variation exhibited among mobile forager societies, focusing especially on evidence for large groups, inequality, large-scale cooperation, and cultivation. We then discuss sedentary and non-egalitarian foragers. We show that such forager societies are far from anomalous, reliably emerging in environments with dense, rich, and predictable resources. Given that humans have occupied and intensively exploited these environments throughout the Late Pleistocene, there is little reason to suspect that they did not correspondingly build societies that were large, hierarchical, and/or (semi-)sedentary by at least 130 ka. We conclude by reviewing implications for the evolutionary understanding of diverse human behaviors.
Limitations of using extant foragers as models of Late Pleistocene societies
The nomadic-egalitarian model was inspired largely by observations of recent foragers11,12,47,48. Such groups, especially those living in Africa such as the Hadza and the Ju/’hoansi (a !Kung-speaking people and one of the “San” or “Bushman” peoples of southern Africa), appear to mostly live in small, mobile bands with relatively egalitarian relations among individuals of similar age and sex49. The conviction that these groups represented the typical forager lifestyle47, and that African hunter-gatherers in particular inhabited an environment similar to the one in which humans spent most of their evolutionary history, motivated the focus on mobile, egalitarian groups. Yet there are major empirical limitations with treating mobile foragers such as the Ju’/hoansi as the primary models of Late Pleistocene societies.
Marginal habitats
The most common criticism of using recent mobile foragers for modeling pre-Holocene societies is that many modern foragers have been pushed to ‘marginal’ or poor-quality habitats by agriculturalists47,48. Because environments appear to be major determinants of forager social organization7, the nomadicegalitarian model can be criticized as reflecting lifeways in a narrow range of harsh environments. Given that Late Pleistocene humans likely lived in both poor- and high-quality habitats, researchers argue, recent forager societies are not representative of the total social diversity that likely characterized the Late Pleistocene.
Two studies have now tested this hypothesis with the Standard Cross-Cultural Sample using net primary productivity as a proxy for habitat quality50,51. Contrary to a widespread expectation, both studies reported no differences in habitat quality between recent hunter-gatherers and agricultural populations. Still, Cunningham et al.51 point out two critical complications:
First, the Standard Cross-Cultural Sample only includes non-industrial societies. It thus underrepresents highly productive aquatic environments such as the Amazon, Ganges, Mississippi, Nile, and Yangtze River valleys and deltas, which have long hosted industrial agricultural societies and were likely appealing to foragers in the past. The published comparisons are thus not between recent foragers and agriculturalists but between recent foragers and the subset of agriculturalists that do not live in industrial societies. Were industrial societies to be included, the analysis would likely provide support to the hypothesis that modern hunter-gatherers live in less productive habitats than agriculturalists.
Second, net primary productivity is a limited, and possibly misleading, proxy for habitat quality. Many of the foragers reported as inhabiting the most productive environments lived in equatorial rainforests, such as the Amazon (Sirionó) and the Congo (Mbuti). While these environments are productive, much of the productivity is stored in non-edible forms, such as woody tissue52. Resources that are edible, meanwhile, are often poisonous or involve high foraging costs, either because they are dispersed, expensive to process, or too high in the canopy to easily access53. Some anthropologists have even argued that, without the help of cultivated foods, a foraging lifestyle would be impossible in tropical rainforests52,54.
Future research will better clarify how the habitats of recent foragers compare with those of agriculturalists. What is clear, however, is that recent hunter-gatherers were excluded from highly productive aquatic environments, such as the Nile and South Africa’s Cape Floral Region, and that popular model populations live in particularly harsh environments.
Other interactions with states and agricultural societies
Agriculturalists have shaped forager social organization in ways beyond just limiting their choice of habitats, further challenging the assumption that modern mobile, egalitarian foragers are models for the past55,56. These interactions have taken many forms, including state incorporation, long-term trade, and slavery. For instance, Marlowe57 noted that Hadza access to iron might back at least 500 years, that their population experienced pre-20th century declines due to the Masai expansion, and that “they were definitely affected by the ivory trade as neighboring groups came to Hadzaland to kill elephants.” Some interactions between foragers and agriculturalists, as in the Philippines and Central African rainforests, go back thousands of years58,59.
The Ju/’hoansi (!Kung)—the people most often used as stand-ins for the Paleolithic31,33— interacted extensively with agriculturalists. Although researchers debate the magnitude and antiquity of the relationships, several points seem less disputed60,61: The Ju/’hoansi began trading with Bantu agriculturalists by at least 500 to 1,500 years ago60. In the 1920s, Bantu agropastoralists entered the Dobe area so that, by the time the Kalahari Research Project was established there in 1962, the Ju/’hoansi were already ensconced in a “larger regional pastoral, tributary, and mercantile economy”60. In 1964, the Dobe Ju/’hoansi numbered 466 individuals across 9 camps62. That same area held 340 Bantu pastoralists and thousands of livestock. In 8 of the 9 camps, Bantu pastoralists and Ju/’hoansi lived together. At any given time, about 20 percent of Ju/’hoansi young men were working with cattle63. Lee recorded little cultivation during his first fieldtrip (1963-1964), although this seems the result of a drought. On his return (1967-1969), he recorded 51% of Ju/’hoansi men planting fields1.
Recent and contemporary foragers are intimately connected with agricultural societies. These relationships impact important features of forager social organization, such as authority, mobility, and corporate group structure, further demonstrating the limitations of relying on mobile and egalitarian foragers as models of pre-Holocene lifeways.
Leadership and authority
The nomadic-egalitarian model implies that decision-making in ancestral societies was through consensus, with a limited role of leadership and authority64. However, this ethnographic pattern may reflect social changes following interactions with agricultural societies which undermine institutions of authority. For example, as foragers become incorporated into larger political entities, outside administrators may monopolize leadership. Or as crucial services, like coordinating warfare or resolving disputes, decline in importance, the need for and approval of leaders may also diminish65,66.
The !Kung experienced one such decline in leadership following the Bantu incursion in the 1920s67. Just what leadership and authority looked like before that time remains unclear, but reports by Fourie68 and Marshall69 both suggest that some positions were hereditary and restricted to men, with the particular norms of heredity varying by area. Although Marshall69,70 was careful to specify that, in the Nyae Nyae area in the 1950s, leaders lacked coercive authority and were more akin to mouthpieces of group consent, Fourie68 wrote that the leader “in fact does exercise considerable influence in the life of the community”. Both wrote that leaders were said to be the true owners of the waterhole, Marshall69 adding that visitors should seek the leader’s permission before taking water.
Mobility
According to the nomadic-egalitarian model, our foraging ancestors had a high degree of mobility, reducing their ability to accrue material wealth and contributing to an egalitarian, decentralized social structure47. Yet many contemporary forager mobility and settlement patterns have been shaped through interactions with large, agricultural societies. Following colonial incorporation and a decreased threat of endemic warfare, large New Guinean fisher-forager communities splintered into smaller groups71. Meanwhile, some peoples lived in small, mobile groups to specialize in the collection and trading of forest products. The Penan of Borneo were long considered “an inordinately primitive hunting and gathering people”72, yet their mobile, foraging lifestyle seemed an adaptation for collecting products considered valuable to Chinese traders, such as rattan, beeswax, and edible birds’ nests72.
Groups might also become mobile to escape political domination. This has been observed among many pastoralist groups, including the Hima of Ankole73, the Yakut Turkmen74, and some pastoral Fulani75, but it likely applies to foragers as well76. In his analyses of upland Southeast Asia and the earliest states in the Levant, Scott77,78 argued that many traits normally considered prototypical of foragers—including not only their mobility and dispersed communities but also non-hereditary social ranking—represent cultural adaptations for escaping state control.
Corporate groups
Several mobile hunter-gatherers, especially popular Paleolithic models such as the !Kung and Hadza, lack systems of corporate groups, such as clans, lineages, and moieties. As a result, some scholars treat corporate groups as complex innovations that developed recently in sociopolitical evolution33. However, the absence of corporate group structure may too reflect interactions with large-scale, agricultural societies. Powerful states might suppress corporate membership to make a populace easier or more productive to govern, such as when the US government unified the clans of the Ifugao people (horticulturalists) after taking control of the Philippines79. Or corporate groups, which commonly function to protect life and property80, may become redundant. By providing the same services, agricultural states may reduce the need for corporate group membership, leading the groups to dissolve with disuse. Finally, the demographic and cultural collapse that results from interacting with agricultural societies, such as through slavery, disease, or rapid acculturation, might also end in the dissolution of corporate social organization81, as seems to have happened with various Tupi-Guarani groups82. Such changes likely occurred among many foragers. The Eastern Pomo, Copper Inuit, and Ju/’hoansi are all coded in the Standard Cross-Cultural Sample as lacking kin-based corporate groups, yet anthropologists studying each of these groups posited that they lost more elaborate social structure following recent interactions with agriculturalist societies67,83,84.
Mischaracterizations of recent foragers
Although many recent and extant foraging groups are assumed to be small-scale, mobile, and egalitarian, such conclusions are often misguided. Instead, recent foragers exhibit considerable variability in group size, mobility, and other features. Insofar as contemporary foragers serve as models for the Pleistocene, their behavior suggests much more variation than the nomadic-egalitarian model permits.
Group size and mobility
Various lines of research suggest that recent mobile hunter-gatherers lived in groups of a few dozen individuals, leading to the conclusion that Paleolithic peoples were similarly small-scale. In a survey of 294 forager societies, Marlowe48 found that the median local group size was 29.5 individuals. Hill et al.5 analyzed field data on a sample of 32 forager societies, including 58 precontact Ache bands and six Ju/’hoansi bands, and reported a weighted mean band size of 28.2 individuals (see also ref. 6). In fact, given that so many comparative studies have converged on an estimated group size of about 25 individuals, Kelly7 has referred to it a “magic number”. Critically, these bands appear to be fluid in composition and nested within larger networks5,18,42.
The focus on mean population sizes hides two important sources of variation. First is withinculture variation. Communities size can vary considerably within a given forager culture, sometimes by as much as an order of magnitude. According to Turnbull’s survey of the Mbuti, group sizes differed dramatically between two Mbuti sub-groups, referred to as the archers (Efe) and net-hunters (Sua Mbuti)85. The archers lived in groups of between 2 and 12 huts, averaging about 6 huts, or 36 individuals, per camp. Net-hunters, meanwhile, lived in groups of between 20 and 40 huts with an average of 25 huts, or 150 individuals, and a maximum of 50 huts, or 250 individuals86. Similarly, Lee recorded the size of 9 !Kung camps in 196462. Excluding visitors and Bantu pastoralists staying in those camps, camp sizes ranged from 9 people at !Kangwa Matse to 117 at /Xai /xai.
The focus on mean population sizes also masks temporal variation. Nomadic foragers in Alaska and northern Australia were both observed to seasonally shift between large, sedentary settlements and dispersed mobile groups43,87,88. Meanwhile, many foragers assembled during serendipitous times and with the purpose of hosting festivities and large-scale ceremonies6. These assemblies commonly numbered in the hundreds, if not low thousands, of individuals and could go on for months, such as occurred among the Warlpiri of Australia89. Even the Ju/’hoansi assembled into larger groups. Not only did several camps share water-holes during years of reduced rainfall90, but people also traditionally held the choma, a 6week-long male initiation which “drew in young men within a radius of 100 km or more” and was gradually phased out at the time Bantu pastoralists moved in67. It remains unclear how many people collected for chomas, although Lee1 speculated that an initiation of 20 or more boys could draw together camps together totaling more than 200 individuals.
Scale of cooperation
The popularity of the nomadic-egalitarian model has led many researchers to conclude that human cooperation was limited to small groups throughout our evolutionary history (e.g., ref. 91). But several lines of research suggest this wasn’t necessarily the case. Boyd and Richerson46 recently reviewed numerous examples of large-scale cooperation among mobile foragers in North America, Australia, Europe, and the Arctic, drawn from archaeological data, historical accounts, and ethnographic descriptions. The examples cover many domains, including communal hunting, construction of shared facilities, and warfare, with cooperative projects often involving hundreds of people, sometimes from neighboring groups. Examples of large-scale cooperation among apparently small-scale foragers appear earlier in the Holocene and even in Pleistocene. Especially striking is evidence of large-scale communal foraging in Middle and Late Pleistocene Europe, including indications of at least two instances of mass buffalo killings c. 400 ka—well before the origins of Homo sapiens92—and the remains of a large number of reindeer at a Middle Paleolithic (c. 54 ka) in Germany93. Another striking example of large-scale cooperation among prehistoric foragers comes from Poverty Point, where an estimated 2,000 laborers and 1,000 supporters cooperated to build Mound A in less than three months (c. 3260 cal. B.P.)94.
Non-egalitarianism
Many apparently mobile, small-scale forager society exhibit coercive authority. Most common is inequality on the basis of age and sex, with a coalition of older men (“elders”) exercising ritual or political authority over other group members (for notable Australian examples, see refs. 95,96). Even when considering individuals of similar age and sex, however, mobile or small-scale foragers exhibit deviations from egalitarianism. Using a sample of 59 societies (including 13 foragers), Garfield et al.97 found that coercive leadership was present among foragers, although less frequent than in other types of societies. Among the Toba of South America’s Gran Chaco, for instance, war chiefs had more wives, were richer, had to be obeyed during war, were stylistically distinguished by “a peculiar arrangement of the hair”, and, with their children, enjoyed “greater esteem than other members of the society”98. Coercive authority has also been documented among peoples living near the Bering Strait99 and among the Khanti of west Siberia, where shamans and elders peoples purportedly “used poor people ‘like slaves’”100. The Warao of northern South America had a “social hierarchy based on political and magico-religious authority”101. Particular individuals were regarded as leaders: They had dominion over the supernatural, imparted orders, and were excluded from hard work, and a leader’s son was said to enjoy “easy access to political leadership”. As these examples demonstrate, shamans and other magico-religious practitioners are especially likely to leverage their supposed supernatural powers to exercise political authority97 (see also refs. 102,103).
Resource management
Researchers have accumulated considerable ethnographic and archaeological evidence suggesting that resource management, such as cultivation and animal management, preceded the Neolithic Revolution. Recent foragers engaged in activities including irrigation, arboriculture, the enhancement of salmon streams, the broadcast sowing of annuals, and the creation of clam gardens104. Holocene foragers managed wild boar populations in Cyprus and Japan before pig domestication105, while archaeological evidence suggests that Melanesian hunter-gatherers were managing populations of cuscuses as early as 20,000 years ago106. Archaeologists reported evidence of intensive plant cultivation at the forager camp Ohalo II in Israel 23,000 years ago—at least 11 millennia before the supposed onset of agriculture in the Near East107. Finally, through controlled fires, the Martu of Australia’s Western Desert generated largescale improvements in habitat quality108,109. Such fire regimes were likely common among foragers elsewhere, including Middle Stone Age hunter-gatherers110. It is no longer clear why we should assume that cultivation, animal management, and other forms of delayed-return resource management developed at the beginning of the Holocene. In fact, indications of domestic-type evolutionary change in wheat and barley at Ohalo II suggest that Late Pleistocene humans may have even incipiently domesticated species, possibly often, well before the Holocene, only to have such evolutionary changes disappear after social and ecological conditions changed107.
The importance of considering sedentary and non-egalitarian foragers
Up to this point, we have focused on populations understood to be mobile and relatively egalitarian. But a large subset of non-agricultural populations clearly violates the nomadic, egalitarian model—those referred to variously as sedentary, hierarchical, or complex hunter-gatherers7.
Evolutionary scholars tend to ignore sedentary or non-egalitarian hunter-gatherers. When Arnold et al.44 examined biological anthropology textbooks published between 2006 and 2014, they found that none mentioned them. Others acknowledge their existence yet reject them as relevant for understanding life in the Late Pleistocene9. Boehm12,111 deliberately excluded them from his database of 150 recent or extant ‘Late-Pleistocene-appropriate’ hunter-gatherer societies. Marlowe48 wrote that sedentary foragers “may not have been rare” immediately before the Holocene, “but for modeling earlier periods we should exclude them”. Lee11 pointed out that sedentary foragers should be discounted when studying the evolution of violence given that small-scale, egalitarian, mobile foragers best represent our evolutionary past.
In contrast, we argue that sedentary hunter-gatherers are relevant for understanding preHolocene lifeways, at least as much as small mobile groups.
Recent examples of sedentary foragers include the Chumash112, New Guinean fisherforagers113,114, and the peoples of the Pacific Northwest115. Such peoples tended to exhibit several common features7: They tended to exploit coastal resources. They sustained very high population densities. Although not necessarily completely sedentary, they exhibited less mobility than classically “nomadic” foragers. They had much larger group sizes, with some villages exceeding 1,000 individuals. They permitted and often institutionalized hierarchy by ceremonially bestowing status upon individuals who accumulated and redistributed surplus. Some groups kept slaves.
Sedentary foragers have demonstrated a profound capacity for building large, politically stratified societies with large-scale cooperation. The Calusa of southern Florida lived in a state or large chiefdom when the Spanish documented them in the mid-1500s. They comprised 50-60 politically consolidated villages along Florida’s southwest coast, although their domain extended from Tampa to Cape Canaveral and down to the Florida Keys, an area larger than modern-day Switzerland116. They collected tribute, centralized power in a hereditary sovereign who ruled for life, supported full-time religious and military specialists, and built large infrastructure projects116,117. Although they appear to have planted some squash and papaya, in addition to managing chile pepper, these constituted trivial contributions to subsistence118; rather, their wealth and surplus derived from rich aquatic resources119.
Scholars—even those who urge that greater attention be paid to sedentary foragers120—have presented at least three reasons why such societies were absent before the Holocene (or the millennia immediately preceding it):
-
They seem anomalous.
-
They seem to rely on aquatic resources—a capacity that, on the basis of archaeological evidence, was believed to develop recently in human history.
-
There is little, if any, archaeological evidence for their existence during the Pleistocene.
These assumptions either have been shown to be wrong or are no longer sufficiently compelling to justify ignoring sedentary foragers in reconstructions of Late Pleistocene human societies.
Sedentary foragers are not anomalous
A longstanding assumption is that sedentary foragers are rare or exceptional, contributing to the conviction that many features of social complexity emerged with agriculture (recently reviewed in ref. 44). Yet this view is no longer viable. Over the past four decades, archival and archaeological research has revealed evidence of sedentary and non-egalitarian hunter-gatherers from all over the world, throughout the Holocene, and even in Pleistocene Europe; see Table 1 and Figure 1 for 34 examples of world regions. As these examples illustrate, many environments that once supported sedentary foragers—such as Japan, the Levant, the Nile River Valley, southern Scandinavia, and the South China Coast—are now inhabited by agriculturalists. Whether this was because these peoples themselves domesticated local species121, they adopted domesticates from neighbors122, or they were demographically displaced, this pattern further suggests that recent hunter-gatherers are underrepresented among particular environments because of agricultural occupation.
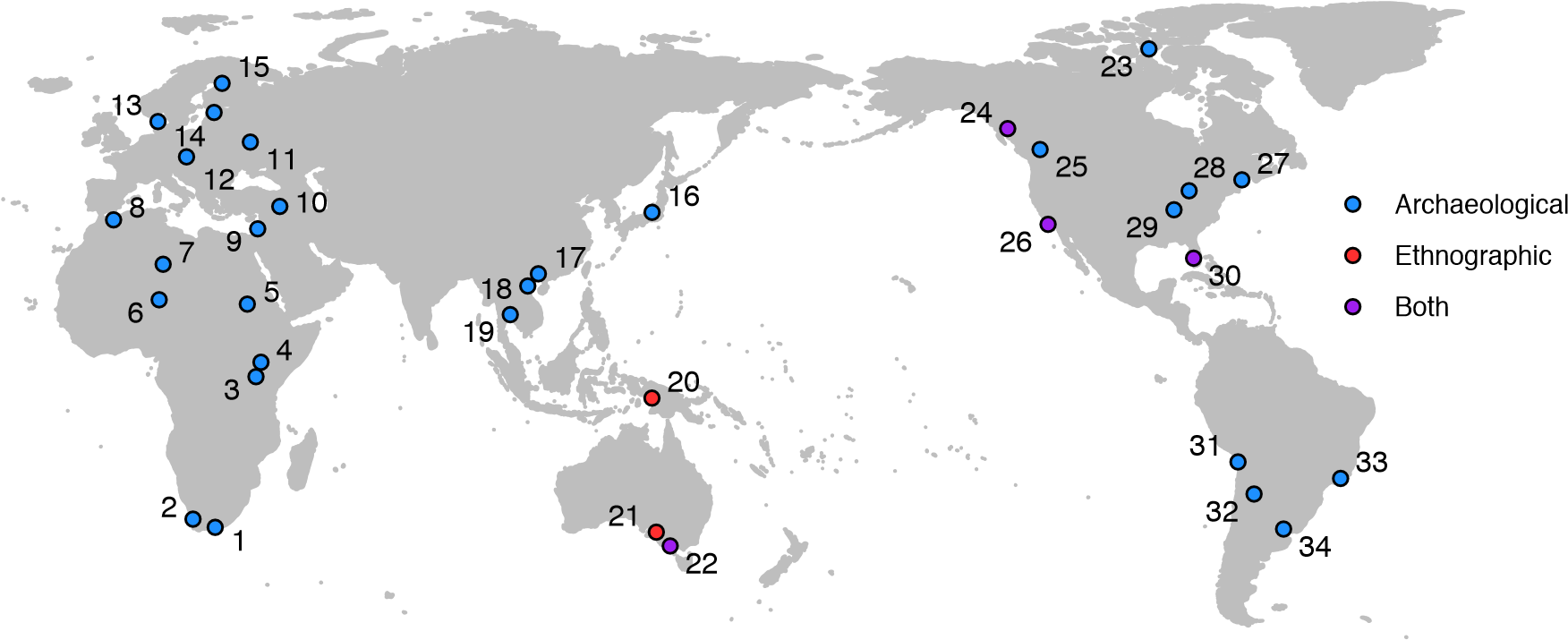
Table 1. Examples of sedentary or semi-sedentary foragers
| 1 | Africa | Southern South Africa Coast (Plettenberg Bay & Cape St. Francis) | 4,500–2,000 BP | Marine resources, including high-trophic-level animals (e.g., seals) | Coastal | X | . | X | . |
| 2 | Africa | Western South Africa Cast (Eland’s Bay & Lambert’s Bay) | 3,000–2,000 BP | Marine resources, especially shellfish | Coastal | X | . | . | . |
| 3 | Africa | Kansyore (Lake Victoria) | 8,000–4,500 cal. BP | Terrestrial and aquatic resources, especially fish | Lacustrine and riverine | X | . | . | . |
| 4 | Africa | Lothagam (Lake Turkana) | 10,000–7,000 BP | Primarily aquatic resources (e.g., Nile perch); terrestrial hunting | Lacustrine | X | . | . | . |
| 5 | Africa | Early Khartoum | 10,000–8,000 cal. BP | Primarily aquatic (riverine) resources | Riparian with floodplains, grasslands, woodlands | X | . | . | . |
| 6 | Africa | Gobero Lake | 9,500–8,200 cal. BP | Terrestrial and lacustrine resources | Lacustrine | X | . | . | . |
| 7 | Africa | Late Acacus | 8,800–8,000 BP | Wild cereals, cattails, barbary sheep | Arid mountains | X | . | . | X |
| 8 | Africa | Taforalt | 13,000–11,000 BP | Diverse terrestrial fauna, esp. land snails, Barbary sheep, and nuts | Arid semi-desert | X | . | . | . |
| 9 | Middle East | Early Natufian | 12,800–11,000 BP | Cereals, legumes, gazelles, cattle, deer | Coastal plain | X | X | X | X |
| 10 | Middle East | Körtik Tepe | 12,300–11,200 cal. BP | Riverine and terrestrial resources (e.g., fish, mammals, plants) | Riverine and open woodland | X | . | . | X |
| 11 | Eurasia | Russian Plain | 18,000–12,000 BP | Terrestrial game, especially large gregarious herbivores (mammoths, bison, horse) | Periglacial steppe; valleys in which megafauna seasonally migrated | X | . | X | . |
| 12 | Eurasia | Pavlovian | 29,000–22,500 BP | Mammoths and other terrestrial resources | Shifting landscape (steppe, shrub, forested) | X | X | . | . |
| 13 | Eurasia | Ertebølle | 6,400–5,900 cal. BP | Marine resources, especially fish | Coastal | X | X | . | . |
| 14 | Eurasia | Bothnian Bay Eastern Coast | 6,500–4,000 cal. BP | Anadromous fish, sea mammals (seals) | Coastal | X | X | X | . |
| 15 | Eurasia | Narva | 7,200–5,900 cal. BP | Diverse aquatic and terrestrial resources, esp. fish | Coastal | X | . | . | . |
| 16 | Eurasia | Jomon (Early period to Final period) | 7,000–2,400 cal. BP | Diverse resources, incl. intensive exploitation of nuts, tubers, and marine resources | Coastal | X | X | X | X |
| ID | Region | Culture/Sub-region | Time | Subsistence | Environment | Reduced mobility | Large groups | Inequality 1 | Resource mgmt. |
| 17 | Eurasia | Dingsishan | 9,000–5,000 BP | Diverse terrestrial and aquatic resources (e.g., fish, shellfish, deer) | Riparian | X | . | . | . |
| 18 | Eurasia | Da But | 6,000–5,500 BP | Fish; mollusks and mammals in swamp and lake environments | Coastal | X | . | . | . |
| 19 | Eurasia | Khok Phanom Di | 4,000–3,500 BP | Estuarine resources (esp. fish, crab, shellfish, turtles) | Coastal | X | . | X | . |
| 20 | Oceania | New Guinean fisherforagers (e.g., Asmat) | 1960 AD | Sago, aquatic resources | Coastal | X | X | X | . |
| 21 | Oceania | Murray River, Australia (e.g., Yaraldi) | 1860 AD | Broad-spectrum (freshwater, marine, and terrestrial resources) | Riparian and lacustrine | X | X | X | X |
| 22 | Oceania | Southwest Victoria, Australia | 2,000 BP–1850 AD | Aquatic wetland resources (esp. eel) & terrestrial plants (e.g., tubers, ferns) | Coastal plain | X | X | X | X |
| 23 | North America | Thule | 1100-1500 AD | Bowhead whale, as well as caribou, fish, seals, and bears | Coastal (warm period) | X | X | X | . |
| 24 | North America | Pacific Northwest Indians (e.g., Tlingit, Haida) | 3,500 BP–1900 AD | Terrestrial and aquatic resources, especially anadromous fish | Coastal | X | X | X | X |
| 25 | North America | Interior Plateau, British Columbia | 2,000–1,000 BP | Terrestrial and aquatic resources, especially anadromous fish | Canyon/river drainage | X | X | X | . |
| 26 | North America | Chumash & ancestors | 6,500 BP–1770 AD | Marine resources, trade with mainland | Coastal islands | X | X | X | X |
| 27 | North America | St. George River Drainage, Maine | 5,000 BP–1650 AD | Shellfish, fish (e.g., cod, swordfish), deer, birds | Coastal | X | . | . | . |
| 28 | North America | Libben | 800–1100 AD | Riparian resources (incl. fish, small mammals, migratory birds)2 | Riparian | X | . | . | . |
| 29 | North America | Indian Knoll | 6,100–4,500 BP | Aquatic and terrestrial resources (e.g., shellfish, deer) | Riparian | X | . | . | . |
| 30 | North America | Calusa | 800–1550 AD | Marine resources and C3 plants (e.g., tree fruits, tubers)1 | Coastal | X | X | X | X |
| 31 | South America | Chinchorro | 7,000–4,000 BP | Marine resources (e.g., fish, sea lions, shellfish); some plants and terrestrial meat | Coastal | X | X | X | . |
| 32 | South America | Puna (high altitude Andean grasslands) | 6,200–3,500 BP | Camelids | Arid high plateau | X | . | X | X |
| 33 | South America | Southeastern coastal Brazil | 4,000–2,000 BP | Marine and some terrestrial resources (e.g., fish, shellfish, tapir, whale, dolphin) | Coastal | X | X | X | . |
| 34 | South America | Plata-Purana Wetlands | 1,700 BP–1500 AD | Wetlands resources (e.g., fish, large rodents, deer, palm) | Coastal wetlands | X | . | . | . |
All cultures or regions varied considerably in social organization. No example listed here exclusively exhibited the noted traits. See Table S1 for details and references.
Humans have exploited aquatic resources deep into the Pleistocene
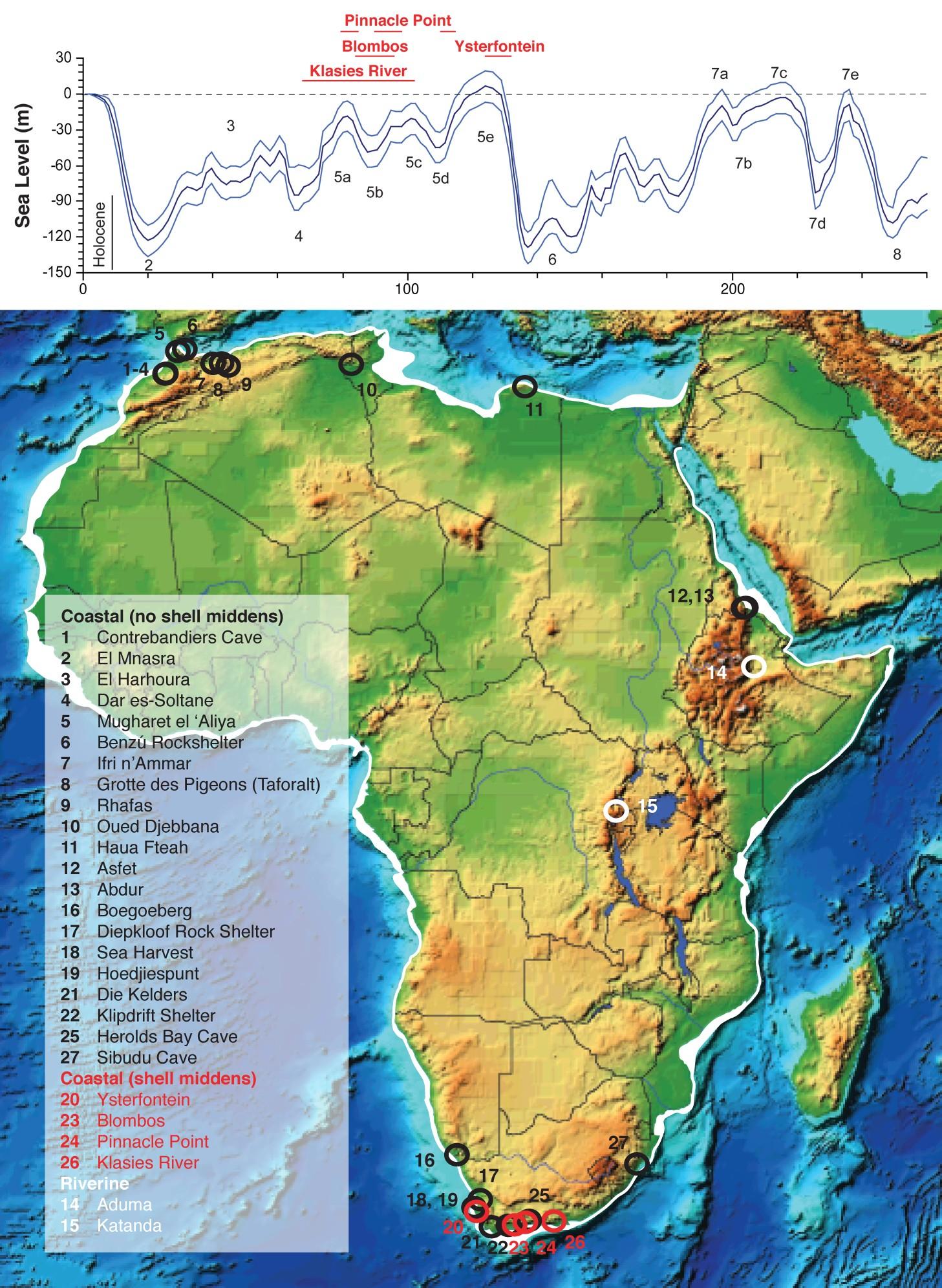
For most of the twentieth century, a common assumption among anthropologists was that humans did not exploit aquatic resources until recently in human history, such as the outset of the Holocene or even later (for a review, see ref. 123). This was based partly on the apparent rarity of evidence of aquatic resource exploitation in the archaeological record124. This assumption no longer holds. Evidence for aquatic resource exploitation goes as far back as 1.95 Ma in northern Kenya125. Archaeologists have discovered shell middens, indicating a commitment to dense and predictable coastal resources, by at least c. 130 ka along the southern African coast126 (Figure 2). There is also evidence that Late Pleistocene humans were systematically exploiting aquatic resources along the north African coast and rivers of Central Africa127 (Figure 2B), and they likely inhabited productive lake margins, such as shoreline sites along Lake Victoria rich in shellfish and aquatic and semi-aquatic plants128. In fact, some scholars now see aquatic (and particularly coastal) adaptation as central for the origin, evolution, and dispersal of modern humans123,127,129.
Even accepting the antiquity of aquatic resource exploitation, it is important to realize that sedentary and non-egalitarian hunter-gatherers do not require aquatic resources. Rather, they seem to emerge in environments with dense, rich, and predictable resources7,135 (although see ref. 136). As Table 1 demonstrates, semi-sedentism and its sociocultural correlates have been documented among foragers subsisting on cereals and sheep, cereals and gazelles, camelids (guanaco and vicuña), mammoths and other large terrestrial herbivores, and bowhead whales.
The evidence for sedentary hunter-gatherers during the Pleistocene
Several archaeological sites provide evidence of sedentary and non-egalitarian hunter-gatherers during the Pleistocene. The most striking are in Upper and Epipaleolithic Europe and include elaborate burials in Sungir (Russia), Arene Candide (Italy), Dolní Věstonice (Czech Republic), Brno 2 (Czech Republic), and Saint-Germaine-la-Rivière (France)137. These burials, many of which are of juveniles, were accompanied by lavish grave goods, such as perforated deer canines and objects made of mammoth ivory. Such goods were often rare or exotic and appeared to require time and mastery to produce—indications of wealth and inequality138,139. Some scholars have gone so far as to argue that the elaborate burial of children in particular suggests inherited status or wealth (e.g., ref. 140). The discovery of circum-Mediterranean sites in which many individuals were buried—known variously as “cemeteries” or “necropolises”—provides further evidence of larger groups, intensive exploitation, and greater sedentism141,142. Again, however, all of these appear at the very end of the Pleistocene.
The archaeological record in Late Pleistocene Africa lacks the conclusive finds of Upper Paleolithic Europe, yet there is still evidence for low-mobility population exploiting the kinds of resources that support large groups, hierarchy, and political complexity. Findings from Late Pleistocene Equatorial Africa, such as 60-70 ka deposits near Lake Edward in the Democratic Republic of the Congo, indicate that populations exploiting dense, predictable aquatic resources lived in communities with low residential mobility (e.g., multi-seasonal occupation with patchily dense artifact accumulations) (reviewed in ref. 128). Archaeologists have uncovered evidence of aquatic resource exploitation in many African Middle Stone Age sites, with indications of systematic marine foraging in South Africa by 160 ka127,134 (Figure 2). Marean143 interprets Middle Stone Age settlements at PP13B (south coast of South Africa) to be residential sites of large social groups that exploited marine resources throughout the year and which exhibited stable and relatively long-term occupations. As Table 1 illustrates, there is strong evidence of semi-sedentary fisher-foragers soon after the Pleistocene-Holocene transition in Africa, such as in the Nile (10 ka), Gobero Lake (9.5 ka), and Lake Turkana (10 ka). There is little reason to presume that such groups would have been rare or non-existent before.
Although large cemeteries, settlements of pit-houses, and elaborate burials with marked social differentiation are absent in the Middle Stone Age record, there are at least three reasons the record is biased against such indications of prehistoric social diversity. First, compared to Europe and North America, far fewer archaeologists have worked in Africa, limiting the opportunity to discover such sites. Second, Africa has fewer caves than Europe and, thus, fewer high-quality preservational environments. Third, and critically, promising sites have likely been submerged or damaged with fluctuating sea levels. Sea-levels today are 120 m higher than at the last glacial maximum, and there were few times in the last 200,000 years when the sea was at or above the present level (Figure 2A). Recognizing these biases, it is of little surprise that some of the best evidence coastal adaptation in Middle Stone Age Africa comes from elevated caves that were both close to ancient coastlines and protected from surging sea levels144. We expect that, with the development of submerged landscape archaeology145, the African archaeological record will yield new discoveries that alter our understanding of social diversity in the Pleistocene.
A new model of Late Pleistocene lifeways
Figure 3 contrasts the nomadic-egalitarian model with what we call the diverse histories model. Both models agree that forager social diversity has declined recently with the spread of agriculture. The models differ, however, in what they posit about social diversity before the Holocene. According to the nomadic-egalitarian model, humans lived predominantly in small-scale, mobile, egalitarian bands before the Holocene. There was minimal diversity across forager societies. In fact, the social diversity observed among recent sedentary and mobile foragers exceeds that of Late Pleistocene hunter-gatherers.
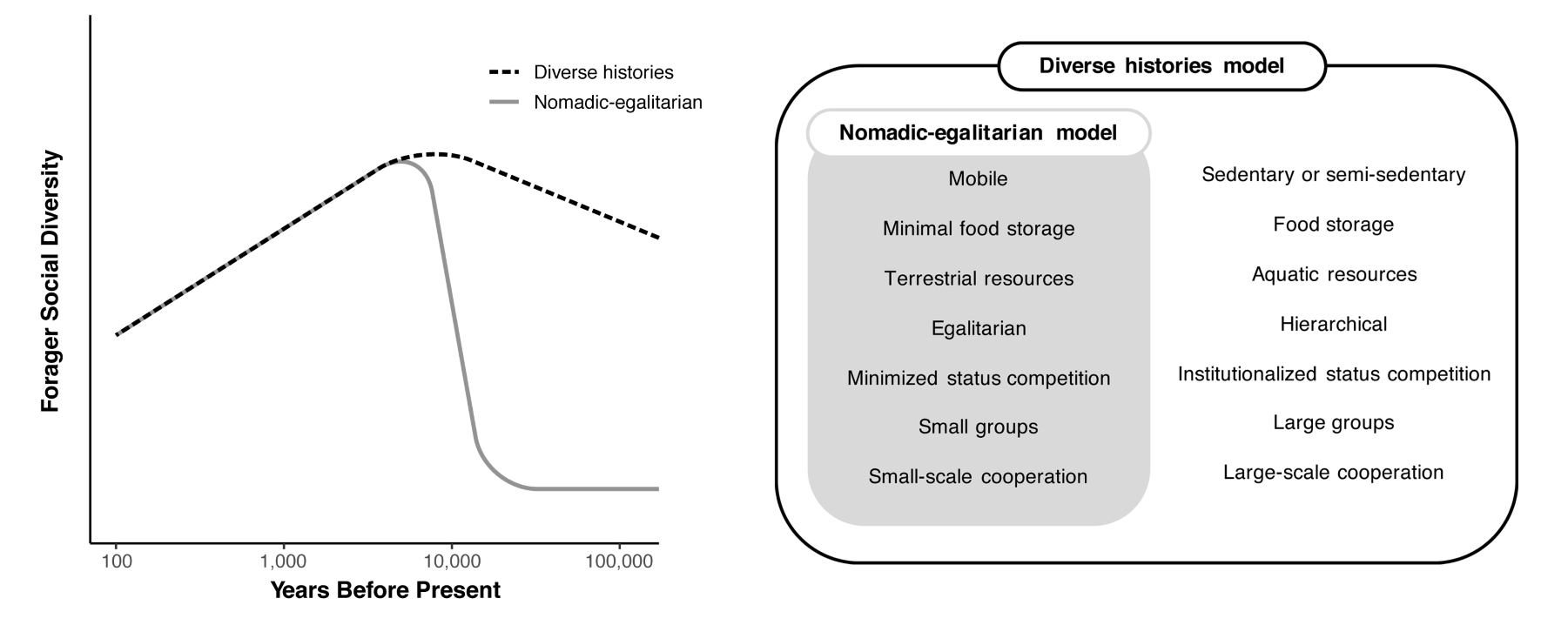
The diverse histories model, in contrast, posits a much higher degree of social diversity throughout the Late Pleistocene. Because behaviorally modern humans likely inhabited many habitats over the last hundred thousand or more years—including not only dry areas like the Kalahari but also consistently productive habitats, such as lake margins, the Nile Valley, or South Africa’s Cape Floral Region—we expect social structures to reflect those diverse ecologies. Just as contemporary foragers living in habitats with dense, predictable resources show a capacity to develop large groups, sedentism, hierarchy, institutionalized authority, and large-scale political consolidation, we expect that pre-Holocene foragers could do the same.
The diverse histories model acknowledges that some humans may have lived in societies similar to contemporary mobile, egalitarian foragers, but posits that these represented one of many common social outcomes. Some foragers would have lived at densities comparable to the Ju/’hoansi (10-16 individuals/100 km2), but others would have lived at densities approaching those of recent coastal foragers like the Chumash (900 individuals/100 km2) or even certain New Guinean groups (2,500 individuals/100 km2)7. Some would have lived in small mobile bands while others would have dwelled in chiefdoms like those in the Pacific Northwest or even, potentially, small tributary states, as with the Calusa of Florida.
These diverse social environments would have represented important habitats for human psychological adaptation. Given the comparably large groups and high densities of foragers living in rich environments, they represent a large proportion of the total human population, even if they take up very little space in a landscape. Imagine, for instance, an expanse with 500 equally sized patches. If 499 were filled with people living at Ju/’hoansi densities and only 1 was inhabited by foragers living at highest densities of coastal New Guinean foragers, still 1 of every 4 individuals would live in the single dense patch.
Implications for the evolution of human behavior
The nomadic-egalitarian model confronts puzzling inconsistencies. On the one hand, our ancestors are said to have spent an appreciable duration of prehistory—anywhere from the last 40,000 to several million years—living in small, egalitarian, mobile bands12,146. Status competition was stifled, and domineering behavior invited censure, ostracism, or execution13,32. This social environment was purportedly critical in shaping the biological foundations of our psychology11,12,48,147: Many scholars argue that understanding the evolution of the human mind requires considering the egalitarian origins of our species12,16,147,148, while others, especially evolutionary psychologists, posit that the cooperation humans exhibit in contemporary large-scale societies reflects adaptations for interacting in small groups of nonstrangers91,149,150.
Yet, as we will show, many reliably developing human behaviors are difficult to explain if the primary or exclusive social environment shaping human psychology was the small-scale, mobile, egalitarian band. Rather, such behaviors become much easier to understand when considered as the products of an evolutionary history involving diverse social environments.
Dominance and other status-seeking behaviors
According to the nomadic-egalitarian model, humans lived in small, egalitarian social groups with little dominance or opportunities for dominance. Moreover, attempts to domineer were presumably met with sanctions such as ostracism or even execution, selecting against such predispositions13,32. Yet, humans everywhere exhibit predispositions to seek and recognize dominance151–153. Even in the egalitarian societies of contemporary foragers, individuals are motivated to domineer each other, creating the demand for the strict sanctions and norms against bullying32,154. The diverse histories model helps explain the persistence of dominance seeking. Humans would have inhabited many environments, including those in which it was possible to accrue resources and wield them for coercive ends. As long as such environments were common enough during our evolutionary history, selection would have favored flexible psychological mechanisms underlying the pursuit and recognition of dominance. A similar argument applies to other status-seeking behaviors, such as the pursuit of prestige (although evolutionary anthropologists have begun to acknowledge the role of prestige competition during our evolutionary past146).
Group identity and minimal group affiliation
From early in development, humans exhibit ingroup biases that are evoked through “mere membership” in a group155,156. Even meaningless or random group assignments—such as on the basis of painting preferences, a coin toss, or shirt color—are sufficient to elicit preferences towards in-group strangers155,157. This is puzzling by the standards of the nomadic-egalitarian model. If, until the last 10,000 years, group sizes were in the dozens and individuals cooperated on a small-scale and rarely with strangers, then it seems unnecessary, even costly, to have psychological predispositions to cooperate with strangers on the basis of arbitrary markers. Under the diverse histories model, however, such psychological predispositions become more understandable. If humans lived in villages of more than a thousand individuals—not uncommon among recent fisher-foragers7,158—and they cooperated at times with hundreds of individuals46, then such prosocial predispositions towards in-group strangers make more evolutionary sense, especially if ancestral groups developed cultural indicators of group membership159.
Intergroup relationships
Using the nomadic-egalitarian model, researchers have advanced opposing arguments about the role of war in human evolution. On the basis of ethnographic descriptions of recent mobile foragers, some conclude that war was a regular feature of human evolution and a major selective force in shaping human psychology28. Others conclude that war was absent during human evolution, appearing only with sedentary and non-egalitarian societies around 10,000 years ago9. Both approaches have difficulty accounting for the flexible range of behaviors seen in contemporary societies: While war continues to be common, many peoples never participate in it160, and groups which formerly engaged in regular warfare quickly abandoned it when social conditions change71. If, however, humans evolved in a variety of social environments—including sedentary settlements, hierarchical societies, and mobile, egalitarian groups— then the frequency and importance of war would have likely varied throughout our evolutionary history. Rather than humans exhibiting a psychology specialized for either peace or war, our diverse evolutionary histories may have endowed us with a flexible behavioral repertoire for interacting with outgroups. Our capacity both to wage war with ease and to abandon it quickly is consistent with humans evolving in diverse social settings involving a range of out-group interactions.
Gene-culture coevolution and human social psychology
An implication of the diverse histories model is a potentially expanded role of gene-culture coevolution in shaping human social psychology. Insofar as (1) cultural evolution allowed humans to build a diversity of societies, and (2) humans either reliably assembled similar societies in similar ecologies or remained in particular societies on temporal scales relevant for genetic evolution, then we would expect culturally evolved features of societies to have been important selection pressures for shaping human psychology. If, for instance, humans regularly developed mechanisms for incentivizing cooperation among large groups of strangers, then our ability to cooperate in modern, large-scale societies may result from adaptations designed for similar institutional settings. If, as we just argued, humans could reliably develop societies on the scale of Californian, Floridian, or New Guinean coastal foragers, then such environments may have selected for predispositions to interact with strangers on the basis of shared group membership rather than individual familiarity. And similarly, if humans lived in societies with substantial social differentiation, such as with economic specialization or defined social classes, then humans may have evolved psychological adaptations for signaling and interpreting those dimensions of class-based identity161. The ease with which we live in contemporary societies dramatically different from small, mobile bands may reflect psychological adaptations designed for similar social ecologies.
Conclusion
We have shown that the empirical foundations of the nomadic-egalitarian model are weak and have a proposed an alternative, the diverse histories model, to replace it. Given (a) the likely diversity of Late Pleistocene environments, (b) the capacity for recent foragers to flexibly build different societies contingent on their ecologies, and (c) the variation in social organization exhibited even among apparently small-scale, mobile foragers, we expect Late Pleistocene social organization to have been much more variable than the nomadic-egalitarian model permits. The diverse histories model helps explain many human behaviors that are puzzling under the prevailing model, including dominance-seeking, minimal group affiliation, and flexible intergroup interactions. Whether or not our alternative model is correct, scholarly reconstructions of Late Pleistocene lifeways require reconsideration.
At two least areas of research will prove valuable for evaluating the diverse histories model. The first is underwater archaeology. Rising sea levels at the end of the Pleistocene submerged the sites most likely to host societies that violate the nomadic-egalitarian model. As techniques of submerged landscape archaeology improve, these previously coastal regions will become critical in determining whether preHolocene societies were non-egalitarian, sedentary, large-scale, and capable of cultivation. Recent discoveries gesture at just how paradigm-shifting these underwater findings may prove to be. Ohalo II in Israel, for instance, was submerged until 1989, when a drop in water levels in the Sea of Galilee exposed it for 10 years. The site has since provided evidence of a 23,000-year-old fisher-forager camp, along with the oldest evidence of plant cultivation and the discovery of hut structures suggesting repeated and prolong occupations107,162.
The second critical area of study is ancient genetics. As the quality and historical depth of genetic samples increases, our ability to make inferences about ancient demographics will improve. Existing research suggests that hunter-gatherer populations today are smaller and more isolated than their Pleistocene predecessors163. Future research with Pleistocene samples will provide more precise characterizations of prehistoric social organization, allowing us to better evaluate the extent to which the diverse histories model describes Late Pleistocene human social organization.
Acknowledgments
We thank J. Compton for his assistance with Figure 2 and D. Pilbeam, M. Price, D. Stibbard-Hawkes, C. Tryon, C. von Rueden, and I. Wallace for their comments on earlier versions of this manuscript. M.S. acknowledges IAST funding from the French National Research Agency (ANR) under the Investments for the Future (Investissements d’Avenir) program, grant ANR-17-EUR-0010.
Competing Interest Statement: The authors declare no competing interests.
References
1. Lee, R. B. The !Kung San: Men, Women, and Work in a Foraging Society. (Cambridge University Press, 1979).
2. Marlowe, F. W. The Hadza: Hunter-gatherers of Tanzania. (University of California Press, 2010).
3. Hill, K. R. & Hurtado, A. M. Aché Life History: The Ecology and Demography of a Foraging People. (Aldine de Gruyter, 1996).
4. Jones, N. G. B., Smith, L. C., O’Connell, J. F., Hawkes, K. & Kamuzora, C. L. Demography of the Hadza, an increasing and high density population of savanna foragers. Am. J. Phys. Anthropol. 89, 159–181 (1992).
5. Hill, K. R. et al. Co-residence patterns in hunter-gatherer societies show unique human social structure. Science 331, 1286–9 (2011).
6. Hamilton, M. J., Milne, B. T., Walker, R. S., Burger, O. & Brown, J. H. The complex structure of hunter-gatherer social networks. Proc. Biol. Sci. 274, 2195–2202 (2007).
7. Kelly, R. L. The lifeways of hunter-gatherers: The foraging spectrum. (2013).
8. Ember, C. R. Myths about hunter-gatherers. Ethnology 17, 439–448 (1978).
9. Fry, D. P., Keith, C. A. & Söderberg, P. Social complexity, inequality and war before farming:
Congruence of comparative forager and archaeological data. in Social inequality before farming? Multidsciplinary approaches to the study of social organization in prehistoric and ethnographic hunter-gatherer-fisher societies (ed. Moreau, L.) 303–320 (McDonald Institute for Archaeological Research, 2020).
10. Fry, D. P. War, peace, and human nature: The challenge of achieving scientific objectivity. in War, peace, and human nature: The convergence of evolutionary and cultural views 1–21 (Oxford University Press, 2013). doi:10.1093/acprof.
11. Lee, R. B. Hunter-gatherers and human evolution: New light on old debates. Annu. Rev. Anthropol. 47, 513–531 (2018).
12. Boehm, C. Moral origins: The evolution of virtue, altruism, and shame. (Basic Books, 2012).
13. Wrangham, R. W. The goodness paradox: The strange relationship between peace and violence in human evolution. (2019).
14. Wrangham, R. W. Two types of aggression in human evolution. Proc. Natl. Acad. Sci. 115, 245–253 (2018).
15. Hrdy, S. B. Mothers and others: The evolutionary understandings of mutual understanding. (The Belknap Press of Harvard University Press, 2009). doi:10.16309/j.cnki.issn.10071776.2003.03.004.
16. Boehm, C. Hierarchy in the Forest. (Harvard University Press, 1999).
17. Apicella, C. L., Marlowe, F. W., Fowler, J. H. & Christakis, N. A. Social networks and cooperation in hunter-gatherers. Nature 481, 497–501 (2012).
18. Hill, K. R., Wood, B. M., Baggio, J., Hurtado, A. M. & Boyd, R. T. Hunter-gatherer inter-band interaction rates: Implications for cumulative culture. PLoS One 9, (2014).
19. Migliano, A. B. et al. Characterization of hunter-gatherer networks and implications for cumulative culture. Nat. Hum. Behav. 1, 1–6 (2017).
20. Migliano, A. B. et al. Hunter-gatherer multilevel sociality accelerates cumulative cultural evolution. Sci. Adv. 6, eaax5913 (2020).
21. von Rueden, C., Gurven, M., Kaplan, H. & Stieglitz, J. Leadership in an egalitarian society. Hum. Nat. 25, 538–66 (2014).
22. Garfield, Z. H., von Rueden, C. & Hagen, E. H. The evolutionary anthropology of political leadership. Leadersh. Q. 30, 59–80 (2019).
23. Boehm, C. A biocultural evolutionary exploration of supernatural sanctioning. in Evolution of religion: Studies, theories, and critiques (eds. Bulbulia, J. et al.) 143–152 (Collins Foundation Press, 2008).
24. Hawkes, K. & Bird, R. B. Showing Off, Handicap Signaling, and the Evolution of Men’s Work. Evol. Anthropol. 11, 58–67 (2002).
25. Smith, D. et al. Cooperation and the evolution of hunter-gatherer storytelling. Nat. Commun. 8, (2017).
26. Wiessner, P. W. Embers of society: Firelight talk among the Ju/’hoansi Bushmen. Proc. Natl. Acad. Sci. 111, 14027–14035 (2014).
27. Knauft, B. M. Violence and Sociality in Human Evolution. Curr. Anthropol. 32, 391 (1991).
28. Wrangham, R. W. & Glowacki, L. Intergroup aggression in chimpanzees and war in nomadic hunter-gatherers: evaluating the chimpanzee model. Hum. Nat. 23, 5–29 (2012).
29. Fry, D. P. & Söderberg, P. Lethal aggression in mobile forager bands and implications for the origins of war. Science 341, 270–3 (2013).
30. Powers, S. T., van Schaik, C. P. & Lehmann, L. How institutions shaped the last major evolutionary transition to large-scale human societies. Philos. Trans. R. Soc. B 371, 20150098 (2016).
31. Johnson, A. W. & Earle, T. The evolution of human societies: From foraging group to agrarian state. (Stanford University Press, 2000).
32. Boehm, C. Egalitarian behavior and reverse dominance hierarchy. Curr. Anthropol. 34, 227–254 (1993).
33. Flannery, K. V. & Marcus, J. The creation of inequality. (Harvard University Press, 2012).
34. Powers, S. T. & Lehmann, L. An evolutionary model explaining the Neolithic transition from egalitarianism to leadership and despotism. Proc. R. Soc. B 281, 20141349 (2014).
35. Bowles, S. & Choi, J.-K. Coevolution of farming and private property during the early Holocene. Proc. Natl. Acad. Sci. U. S. A. 110, 8830–5 (2013).
36. Bowles, S. & Choi, J. K. The Neolithic agricultural revolution and the origins of private property. J. Polit. Econ. 127, 2186–2228 (2019).
37. Hartley, T. The continuing evolution of ownership. PLoS One 14, e0211871 (2019).
38. Richerson, P. J., Boyd, R. & Bettinger, R. L. Was agriculture impossible during the Pleistocene but mandatory during the Holocene? A climate change hypothesis. Am. Antiq. 66, 387–411 (2001).
39. Bellwood, P. First farmers: The origins of agricultural societies. (Blackwell Publishing, 2005).
40. Fukuyama, F. The origins of political order: From prehuman times to the French Revolution. (Farrar, Straus and Giroux, 2011).
41. Lewin, R. New views emerge on hunters and gatherers. Science (80-. ). 240, 1146–1148 (1988).
42. Bird, D. W., Bird, R. B., Codding, B. F. & Zeanah, D. W. Variability in the organization and size of hunter-gatherer groups: Foragers do not live in small-scale societies. J. Hum. Evol. 131, 96–108 (2019).
43. Wengrow, D. & Graeber, D. Farewell to the ‘childhood of man’: Ritual, seasonality, and the origins of inequality. J. R. Anthropol. Inst. 21, 597–619 (2015).
44. Arnold, J. E. et al. Entrenched Disbelief: Complex Hunter-Gatherers and the Case for Inclusive Cultural Evolutionary Thinking. J. Archaeol. Method Theory 23, 448–499 (2016).
45. Social inequality before farming? Multidisciplinary approaches to the study of social organization in prehistoric and ethnographic hunter-gatherer-fisher societies. (McDonald Institute for Archaeological Research, 2020).
46. Boyd, R. & Richerson, P. J. Large-scale cooperation in small-scale foraging societies.
47. Lee, R. B. & DeVore, I. Problems in the study of hunters and gatherers. in Man the hunter (eds. Lee, R. B. & DeVore, I.) 3–12 (1968).
48. Marlowe, F. W. Hunter-gatherers and human evolution. Evol. Anthropol. 14, 54–67 (2005).
49. Ember, C. R. Hunter-gatherers. in Explaining human culture (ed. Ember, C. R.) (Human Relations Area Files, 2020).
50. Porter, C. C. & Marlowe, F. W. How marginal are forager habitats? J. Archaeol. Sci. 34, 59–68 (2007).
51. Cunningham, A. J., Worthington, S., Venkataraman, V. V. & Wrangham, R. W. Do modern huntergatherers live in marginal habitats? J. Archaeol. Sci. Reports 25, 584–599 (2019).
52. Bailey, R. C. et al. Hunting and gathering in tropical rain forest: Is it possible? Am. Anthropol. 91, 59–82 (1989).
53. Headland, T. N. The wild yam question: How well could independent hunter-gatherers live in a tropical rain forest ecosystem? Hum. Ecol. 15, 463–491 (1987).
54. Headland, T. N. & Bailey, R. C. Introduction: Have hunter-gatherers ever lived in tropical rain forest independently of agriculture? Hum. Ecol. 19, 115–122 (1991).
55. Headland, T. N. & Reid, L. A. Hunter-gatherers and their neighbors from prehistory to the present. Curr. Anthropol. 30, 43–66 (1989).
56. Hunter-gatherers in a changing world. Hunter-Gatherers in a Changing World (Springer, 2016). doi:10.1007/978-3-319-42271-8.
57. Marlowe, F. W. The Hadza: Hunter-gatherers of Tanzania. (University of California Press, 2010).
58. Junker, L. L. Economic specialization and inter-ethnic trade between foragers and farmers in the prehispanic Philippines. in Forager-traders in South and Southeast Asia (eds. Morrison, K. D. & Junker, L. L.) 203–241 (Cambridge University Press, 2002).
59. Verdu, P. et al. Origins and genetic diversity of pygmy hunter-gatherers from Western Central Africa. Curr. Biol. 19, 312–318 (2009).
60. Solway, J. S. & Lee, R. B. Foragers, genuine or spurious? Curr. Anthropol. 31, 109–146 (1990).
61. Wilmsen, E. N. & Denbow, J. R. Paradigm history of San-speaking peoples and current attempts at revision. Curr. Anthropol. 31, 489–524 (1990).
62. Lee, R. B. The Dobe !Kung. (Holt, Rinehart, and Winston, Inc., 1984).
63. Lee, R. B. Introduction. in Kalahari hunter-gatherers: Studies of the !Kung San and their neighbors (eds. Lee, R. B. & DeVore, I.) 1–24 (Harvard University Press, 1976).
64. Boehm, C. Impact of the human egalitarian syndrome on Darwinian selection mechanics. Am. Nat. 150, 100–121 (1997).
65. Glowacki, L. & von Rueden, C. Leadership solves collective action problems in small-scale societies. Philos. Trans. R. Soc. B 370, 20150010 (2015).
66. Garfield, Z. H., Hubbard, R. L. & Hagen, E. H. Evolutionary models of leadership: Tests and synthesis. Hum. Nat. 30, 23–58 (2019).
67. Wiessner, P. Pathways of the past: !Kung San hxaro exchange and history. in Überlebensstrategien in Afrika (eds. Bollig, M. & Klees, F.) 101–124 (Heinrich-Barth Institut, 1994).
68. Fourie, L. The Bushmen of south west Africa. (Cape Times, 1928).
69. Marshall, L. The !Kung Bushmen of the Kalahari Desert. in Peoples of Africa (ed. Gibbs, Jr., J. L.) 243–278 (Holt, Rinehart, and Winston, Inc., 1965).
70. Marshall, L. The !Kung of Nyae Nyae. (Harvard University Press, 1976).
71. Roscoe, P. The fortunes of foragers in colonial and post-colonial New Guinea. in Hunter-gatherers in a changing world (eds. Reyes-García, V. & Pyhälä, A.) (Springer, 2016).
72. Hoffman, C. L. Punan foragers in the trading networks of Southeast Asia. in Past and present in hunter-gatherer studies (ed. Carmel Shrire) 123–149 (Academic Press, 1984).
73. Elam, Y. Nomadism in Ankole as a substitute for rebellion. Africa (Lond). 49, 147–158 (1979).
74. Irons, W. Nomadism as a political adaptation: The case of the Yomut Turkmen. Am. Ethnol. 1, 635–658 (1974).
75. Burnham, P. ‘Regroupement’ and mobile societies: Two Cameroon cases. J. Afr. Hist. 16, 577–594 (1975).
76. Rambo, A. T. Why are the Semang? Ecology and ethnogenesis of aboriginal groups in peninsular Malaysia. in Ethnic diversity and the control of natural resources in Southeast Asia (eds. Rambo, A. T., Gollogly, K. & Hutterer, K.) (Center for South and Southeast Asia, 1988).
77. Scott, J. C. The art of not being governed: An anarchist history of upland Southeast Asia. (2009). doi:10.1080/10848770.2013.791444.
78. Scott, J. C. Against the grain: A deep history of the earliest states. (Yale University Press, 2017).
79. Beyer, H. O. & Barton, R. F. An Ifugao burial ceremony. Philipp. J. Sci. 6, 227–252 (1911).
80. Glowacki, L. The emergence of locally adaptive institutions: Insights from traditional social structures of East African pastoralists. BioSystems 198, 104257 (2020).
81. Service, E. R. Primitive social organization: An evolutionary perspective. (Random House, 1971).
82. Walker, R. S., Wichmann, S., Mailund, T. & Atkisson, C. J. Cultural phylogenetics of the Tupi language family in lowland South America. PLoS One 7, e35025 (2012).
83. Gifford, E. W. Pomo lands on Clear Lake. Univ. Calif. Publ. Am. Archaeol. Ethnol. 20, 77–92 (1923).
84. Condon, R. G. Inuit behavior and seasonal change in the Canadian Arctic. (UMI Research Press, 1983).
85. Turnbull, C. M. The Mbuti Pygmies: An Ethnographic Survey. Anthropological Papers of the American Museum of Natural History vol. 50 (The American Museum of Natural History, 1965).
86. Putnam, P. The Pygmies of the Ituri Forest. in A reader in general anthropology (ed. Carleton S. Coon) 322–342 (Henry Holt and Company, 1948).
87. White, C. & Peterson, N. Ethnographic Interpretations of the Prehistory of Western Arnhem Land. Southwest. J. Anthropol. 25, 45–67 (1969).
88. Mauss, M. Seasonal variations of the Eskimo: A study in social morphology. (Routledge & Kegan Paul, 1950).
89. Meggitt, M. J. Desert people: A study of the Walbiri aborigines of central Australia. (Angus and Robertson, 1974).
90. Lee, R. B. !Kung spatial organization: An ecological and historical perspective. Hum. Ecol. 1, 125–147 (1972).
91. Tooby, J. & Cosmides, L. Human cooperation shows the distinctive signatures of adaptations to small-scale social life. Behav. Brain Sci. 39, e54 (2016).
92. Rodríguez-Hidalgo, A. et al. Human predatory behavior and the social implications of communal hunting based on evidence from the TD10.2 bison bone bed at Gran Dolina (Atapuerca, Spain). J. Hum. Evol. 105, 89–122 (2017).
93. Gaudzinski, S. & Roebroeks, W. Adults only. Reindeer hunting at the Middle Palaeolithic site Salzgitter Lebenstedt, northern Germany. J. Hum. Evol. 38, 497–521 (2000).
94. Ortmann, A. L. & Kidder, T. R. Building Mound A at Poverty Point, Louisiana: Monumental public architecture, ritual practice, and implications for hunter-gatherer complexity. Geoarchaeology 28, 66–86 (2013).
95. Warner, W. L. A black civilization: A social study of an Australian tribe. (Harper & Brothers, 1958).
96. Hart, C. W. M. & Pilling, A. R. The Tiwi of north Australia. (Holt, Rinehart, and Winston, Inc., 1960).
97. Garfield, Z. H., Syme, K. L. & Hagen, E. H. Universal and variable leadership dimensions across human societies. Evol. Hum. Behav. 41, 397–414 (2020).
98. Karsten, R. Indian tribes of the Argentine and Bolivian Chaco: Ethnological studies. (Akademische Buchhandlung, 1932).
99. Nelson, E. W. The Eskimo about Bering Strait. (Government Printing Office, 1900).
100. Bartels, D. & Bartels, A. Khanti domestic and political organization. in The Cambridge encyclopedia of hunters and gatherers (eds. Lee, R. B. & Richard Daly) 164–165 (Cambridge University Press, 1999).
101. Suárez, M. M. The Warao: Natives of the Orinoco Delta. (Human Relations Area Files, 2000).
102. Singh, M. The cultural evolution of shamanism. Behav. Brain Sci. 41, e66 (2018).
103. Singh, M. & Henrich, J. Why do religious leaders observe costly prohibitions? Examining taboos on Mentawai shamans. Evol. Hum. Sci. 2, e32 (2020).
104. Smith, B. D. General patterns of niche construction and the management of ‘wild’ plant and animal resources by small-scale pre-industrial societies. Philos. Trans. R. Soc. B Biol. Sci. 366, 836–848 (2011).
105. Price, M. & Hongo, H. The archaeology of pig domestication in Eurasia. J. Archaeol. Res. (2019) doi:10.1007/s10814-019-09142-9.
106. Heinsohn, T. E. Marsupials as introduced species: Long-term anthropogenic expansion of the marsupial frontier and its implications for zoogeographic interpretation. Altered Ecol. (Terra Aust.
32) Fire, Clim. Hum. Influ. Terr. landscapes (2010) doi:10.22459/ta32.11.2010.09.
107. Snir, A. et al. The origin of cultivation and proto-weeds, long before neolithic farming. PLoS One 10, 1–12 (2015).
108. Bliege Bird, R. et al. Fire mosaics and habitat choice in nomadic foragers. Proc. Natl. Acad. Sci. U. S. A. 117, 12904–12914 (2020).
109. Bliege Bird, R., Bird, D. W., Codding, B. F., Parker, C. H. & Jones, J. H. The ‘fire stick farming’ hypothesis: Australian Aboriginal foraging strategies, biodiversity, and anthropogenic fire mosaics. Proc. Natl. Acad. Sci. U. S. A. 105, 14796–14801 (2008).
110. Thompson, J. C., Wright, D. K. & Ivory, S. J. The emergence and intensification of early huntergatherer niche construction. Evol. Anthropol. (2020).
111. Boehm, C. Purposive social selection and the evolution of human altruism. Cross-Cultural Res. 42, 319–352 (2008).
112. Arnold, J. E. Complex hunter-gatherer-fishers of prehistoric California: Chiefs, specialists, and maritime adaptations of the Channel Islands. Am. Antiq. 57, 60–84 (1992).
113. Roscoe, P. The hunters and gatherers of New Guinea. Curr. Anthropol. 43, 153–162 (2002).
114. Roscoe, P. Fish, game, and the foundations of complexity in forager society: The evidence from New Guinea. Cross-Cultural Res. 40, 29–46 (2006).
115. Ames, K. M. The Northwest Coast: Complex hunter-gatherers, ecology, and social evolution. Annu. Rev. Anthropol. 23, 209–229 (1994).
116. Thompson, V. D., Marquardt, W. H., Walker, K. J., Thompson, A. D. R. & Newsom, L. A. Collective action, state building, and the rise of the Calusa, southwest Florida, USA. J. Anthropol. Archaeol. 51, 28–44 (2018).
117. Thompson, V. D. et al. Ancient engineering of fish capture and storage in southwest Florida. Proc. Natl. Acad. Sci. U. S. A. 117, 8374–8381 (2020).
118. Hutchinson, D. L. et al. The Calusa and prehistoric subsistence in central and south Gulf Coast Florida. J. Anthropol. Archaeol. 41, 55–73 (2016).
119. Marquardt, W. H. Tracking the Calusa: A retrospective. Southeast. Archaeol. 33, 1–24 (2014).
120. Prehistoric hunter-gatherers: The emergence of complexity. (Academic Press, Inc., 1985).
121. Maher, L. A., Richter, T. & Stock, J. T. The Pre-Natufian Epipaleolithic: Long-term Behavioral Trends in the Levant. Evol. Anthropol. 21, 69–81 (2012).
122. Lee, J.-J. From shellfish gathering to agriculture in prehistoric Korea: The Chulmun to Mumun transition. (University of Wisconsin-Madison, 2001).
123. Erlandson, J. M. The archaeology of aquatic adaptations: Paradigms for a new millennium. J. Archaeol. Res. 9, 287–350 (2001).
124. Osborn, A. J. Strandloopers, Mermaids, and Other Fairytales; Ecological Determinants of Marine Resource Utilization - The Peruvian Case. in For Theory Building in Archaeology 157–205 (1977).
125. Braun, D. R. et al. Early hominin diet included diverse terrestrial and aquatic animals 1.95 Ma in East Turkana, Kenya. Proc. Natl. Acad. Sci. U. S. A. 107, 10002–10007 (2010).
126. Marean, C. W. The origins and significance of coastal resource use in Africa and Western Eurasia. J. Hum. Evol. 77, 17–40 (2014).
127. Marean, C. W. The transition to foraging for dense and predictable resources and its impact on the evolution of modern humans. Philos. Trans. R. Soc. B Biol. Sci. 371, (2016).
128. Tryon, C. A. et al. The Pleistocene prehistory of the Lake Victoria basin. Quat. Int. 404, 100–114 (2016).
129. Erlandson, J. M. et al. The kelp highway hypothesis: Marine ecology, the coastal migration theory, and the peopling of the Americas. J. Isl. Coast. Archaeol. 2, 161–174 (2007).
130. Waelbroeck, C. et al. Sea-level and deep water temperature changes derived from benthic foraminifera isotopic records. Quat. Sci. Rev. 21, 295–305 (2002).
131. Will, M., Kandel, A. W., Kyriacou, K. & Conard, N. J. An evolutionary perspective on coastal adaptations by modern humans during the Middle Stone Age of Africa. Quat. Int. 404, 68–86 (2016).
132. Compton, J. S. Pleistocene sea-level fluctuations and human evolution on the southern coastal plain of South Africa. Quarternary Sci. Rev. 30, 506–527 (2011).
133. Yellen, J. E. et al. The archaeology of Aduma Middle Stone Age sites in the Awash Valley, Ethiopia. PaleoAnthropology 2005, 25–100 (2005).
134. McBrearty, S. & Brooks, A. S. The revolution that wasn’t: a new interpretation of the origin of modern human behavior. J. Hum. Evol. 39, 453–563 (2000).
135. Dyson-Hudson, R. & Smith, E. A. Human Teritoriality: An Ecological Reassessment. Am. Anthropol. 80, 21–41 (1978).
136. Jeffrey, J. L. & Lahr, M. M. Exploring fisher-forager complexity in an African context. in Social inequality before farming? Multidsciplinary approaches to the study of social organization in prehistoric and ethnographic hunter-gatherer-fisher societies (ed. Moreau, L.) 255–278 (McDonald Institute for Archaeological Research, 2020).
137. Pettitt, P. The Palaeolithic origins of human burial. The Palaeolithic Origins of Human Burial (Routledge, 2010). doi:10.4324/9780203813300.
138. D’Errico, F. & Vanhaeren, M. Upper Paleolithic mortuary practices: Reflection of ethnic affiliation, social complexity, and cultural turnover. Death Ritual. Soc. Order Archaeol. Immortal. Anc. World 45–62 (2015) doi:10.1017/cbo9781316014509.005.
139. Vanhaeren, M. & d’Errico, F. Grave goods from the Saint-Germain-la-Rivière burial: Evidence for social inequality in the Upper Palaeolithic. J. Anthropol. Archaeol. 24, 117–134 (2005).
140. Scheidel, W. The great leveler: Violence and the history of inequality from the stone age to the twenty-first century. (Princeton University Press, 2017).
141. Formicola, V., Pettitt, P. B., Maggi, R. & Hedges, R. Tempo and mode of formation of the Late Epigravettian necropolis of Arene Candide cave (Italy): Direct radiocarbon evidence. J. Archaeol. Sci. 32, 1598–1602 (2005).
142. Barton, R. N. E., Bouzouggar, A., Collcutt, S. N. & Humphries, L. T. Cemeteries and sedentism in the later Stone Age of NW Africa: Excavations at Grotte des Pigeons, Taforalt, Morocco. (Römiisch Germanisches Zentralmuseum, 2019).
143. Marean, C. W. Pinnacle Point Cave 13B (Western Cape Province, South Africa) in context: The Cape Floral kingdom, shellfish, and modern human origins. J. Hum. Evol. 59, 425–443 (2010).
144. Marean, C. W. When the sea saved humanity. Sci. Am. 303, 55–61 (2010).
145. Bailey, G. N. & Flemming, N. C. Archaeology of the continental shelf: Marine resources, submerged landscapes and underwater archaeology. Quat. Sci. Rev. 27, 2153–2165 (2008).
146. von Rueden, C. Making and unmaking egalitarianism in small-scale human societies. Curr. Opin. Psychol. 33, 167–171 (2020).
147. Kaplan, H. S., Hooper, P. L. & Gurven, M. The evolutionary and ecological roots of human social organization. Philos. Trans. R. Soc. B Biol. Sci. 364, 3289–3299 (2009).
148. Whiten, A. & Erdal, D. The human socio-cognitive niche and its evolutionary origins. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 367, 2119–29 (2012).
149. Krasnow, M. M., Delton, A. W., Tooby, J. & Cosmides, L. Meeting now suggests we will meet again: Implications for debates on the evolution of cooperation. Sci. Rep. 3, 1–8 (2013).
150. Delton, A. W., Krasnow, M. M., Cosmides, L. & Tooby, J. Evolution of direct reciprocity under uncertainty can explain human generosity in one-shot encounters. Proc. Natl. Acad. Sci. U. S. A. 108, 13335–13340 (2011).
151. Johnson, S. L., Leedom, L. J. & Muhtadie, L. The dominance behavioral system and psychopathology: Evidence from self-report, observational, and biological studies. Psychol. Bull. 138, 692–743 (2012).
152. Gazes, R. P., Hampton, R. R. & Lourenco, S. F. Transitive inference of social dominance by human infants. Dev. Sci. 20, 1–10 (2017).
153. Charafeddine, R. et al. How Preschoolers Use Cues of Dominance to Make Sense of Their Social Environment. J. Cogn. Dev. 16, 587–607 (2015).
154. Wiessner, P. Norm enforcement among the Ju/’hoansi Bushmen. Hum. Nat. 16, 115–145 (2005).
155. Dunham, Y. Mere Membership. Trends Cogn. Sci. 22, 780–793 (2018).
156. Richter, N., Over, H. & Dunham, Y. The Effects of Minimal Group Membership on Young
Preschoolers’ Social Preferences, Estimates of Similarity, and Behavioral Attribution. Collabra 2, 8 (2016).
157. Mullen, B., Brown, R. & Smith, C. Ingroup bias as a function of salience, relevance, and status: An integration. Eur. J. Soc. Psychol. 22, 103–122 (1992).
158. Knauft, B. M. South coast New Guinea cultures: History, comparison, dialectic. (Cambridge University Press, 1993). doi:10.16309/j.cnki.issn.1007-1776.2003.03.004.
159. McElreath, R., Boyd, R. & Richerson, P. J. Shared Norms and the Evolution of Ethnic Markers. Curr. Anthropol. 44, 122–129 (2003).
160. Fry, D. P. Beyond war: The human potential for peace. (Oxford University Press, 2007).
161. Smaldino, P. E. Social identity and cooperation in cultural evolution. Behav. Processes 161, 108–116 (2019).
162. Nadel, D. et al. Stone Age hut in Israel yields world’s oldest evidence of bedding. Proc. Natl. Acad. Sci. U. S. A. 101, 6821–6826 (2004).
163. Bergström, A. et al. Insights into human genetic variation and population history from 929 diverse genomes. Science (80-. ). 367, (2020).
Table S1. Details and references for Table 1.
| ID | Region | Culture/Sub- region | Time | Subsistence | Environment | Reduced mobility | Large groups | Inequality1 | Resource management | References | |
| 1 | Africa | Southern South Africa Coast (Plettenberg Bay & Cape St. Francis) | 4,500-2,000 BP | Marine resources, including high- trophic-level animals (e.g., seals) | Coastal | Consistent dietary differences between nearby groups suggest reduced mobility | Burials vary in quantity and type of grave goods; Klasies River site includes richly adorned infant burial | (Hall and Binneman 1987; Sealy 2006) | |||
| 2 | Africa | Western South Africa Cast (Eland's Bay & Lambert's Bay) | 3,000-2,000 BP | Marine resources, especially shellfish | Coastal | Reduced use of exotic resources; shift away from mobile prey | (Jerardino 2012; Jerardino and Navarro 2018) | ||||
| 3 | Africa | Kansyore (Lake Victoria) | 8,000-4,500 cal. BP | Terrestrial and aquatic resources, especially fish | Lacustrine and riverine | Semi-sedentism posited for riverside sites based on site size and density of artifacts | (Prendergast I 2010) | ||||
| 4 | Africa | Lothagam (Lake Turkana) | 10,000-7,000 BP | Primarily aquatic resources (e.g., Nile perch); terrestrial hunting | Lacustrine | Cemetery | (Angel et al. 1980) I | ||||
| 5 | Africa | Early Khartoum | 10,000-8,000 cal. BP | ||||||||
| Primarily aquatic (riverine) resources | Riparian with floodplains, grasslands, woodlands | Pottery, thick archaeological deposits, high density of burials and potsherds, reconstructions of animal exploitation, storage (salting fish) | (Garcea 2006; Maritan et al. 2018; Salvator! 2012; Salvator! and Usa! 2019) I | ||||||||
| 6 | Africa | Gobero Lake | 9,500-8,200 cal. BP | Terrestrial and lacustrine resources (e.g., Nile perch) | Lacustrine | Radiogenic strontium isotopic ratio variability lower than other HGs thought to be sedentary; burial density, groundstones | (Sereno et al. 2008; Stojanowski and Knudson 2011) | ||||
| 7 | Africa | Late Acacus | 8,800-8,000 BP | Wild cereals, cattails, Barbary sheep | Arid mountains | Management of barbary sheep (penning, feeding) | (Garcea 2006) | Wooden hut, grindstones, pottery, sheep penning | |||
| 8 | Africa | Taforalt | 13,000-11,000 BP | Diverse terrestrial fauna, esp. land snails, Barbary sheep, and nuts | Arid semidesert | (Barton et al. 2019; Hogue 2014) “ | Cemetery; persistent occupation of sites and occupation for much of annual cycle |
| ID | Region | Culture/Sub- region | Time | Subsistence | Environment | Reduced mobility | Large groups | Inequality1 | Resource management | References |
| 9 | Middle East | Early Natufian | 12,500-11,000 BP | Cereals, legumes, gazelles, cattle, deer | Coastal plain | Stone architecture; heavy groundstone artifacts; cemeteries; commensals | Large settlements | Small proportion of adorned skeletons | Cereal cultivation? | (Bar-Yosef 1994; Belfer-Cohen and Bar-Yosef 2000; T. D. Price and Bar- Yosef 2010) |
| 10 | Middle East | Kortik Tepe | 12,500-11,200 cal. BP | Riverine and terrestrial resources (e.g., fish, mammals, plants) | Riverine and open woodland | Permanent buildings, multiple occupation layers, large grinding stones and mortars | Cultivation of cereals and pulses | (Rossner et al. 2018) | ||
| 11 | Eurasia | Russian Plain | 18,000-12,000 BP | Terrestrial game, especially large gregarious herbivores (mammoths, bison, horse) | Periglacial steppe; valleys in which megafauna seasonally migrated | Storage pits, mammoth bone dwellings | Dwellings vary with respect to size and number of storage pits and elaborate goods | (Soffer 1985, 1989) | ||
| 12 | Eurasia | Pavlovian | 29,000-22,500 BP | Mammoths and other terrestrial resources | Shifting landscape (steppe, shrub, forested) | Intensity of occupation; stability of dwellings; delicate technologies; familiarity with obesity | Large settlements estimated at 100 individuals | (Svoboda 2016; Trinkaus 2005; Trinkaus and Svoboda 2006) | ||
| 13 | Eurasia | Ertebolle | 6,400-5,900 cal. BP | Marine resources, especially fish | Coastal | Cemeteries; analyses of middens | Large settlements | (T. D. Price 2015; Rowley-Conwy 1999) | ||
| 14 | Eurasia | Bothnian Bay Eastern Coast | 6,500-4,000 cal. BP | Anadromous fish, sea mammals (seals) | Coastal | Megastructures, house pit sites, stationary fishing structures | Large house pit sites | Evidence of social differentiation among corporate “houses” | (Tallavaara and Pesonen 2020; Vaneeckhout 2010) | |
| 15 | Eurasia | Narva | 7,200-5,900 cal. BP | Diverse aquatic and terrestrial resources, esp. fish | Coastal | Pottery, evidence of multi-season occupation | (Kriiska et al. 2017; Oras etal. 2017) | |||
| 16 | Eurasia | Jomon (Early period to Final period) | 7,000-2,400 cal. BP | Diverse resources, incl. intensive exploitation of nuts, tubers, and marine resources | Coastal | Settlements with pit-dwellings | Some extremely large settlements | Burial data from Late and Final periods in Tohoku indicates hereditary inequality | Managed wild boar; evidence of management of chestnut, lacquer tree, hemp, even soybean and azuki bean | (Habu 2004; Nasu and Momohara 2016; M. Price and Hongo 2019) |
| 17 | Eurasia | Dingsishan | 9,000-5,000 BP | Diverse terrestrial and aquatic resources (e.g., fish, shellfish, | Riparian | Cemeteries, pottery manufacturing workshops | (Chi and Hung 2012; Xianguo 2002) | |||
| 18 | Eurasia | Da But | 6,000-5,500 BP | deer) Fish; mollusks and mammals | Coastal | Cemeteries | (Viet 2005) |
| ID | Region | Culture/Sub- region | Time | Subsistence | Environment | Reduced mobility | Large groups | Inequality1 | Resource management | References |
| in swamp and lake environments | ||||||||||
| 19 | Eurasia | Khok Phanom Di | 4,000-3,500 BP | Estuarine resources (esp. fish, crab, shellfish, turtles) | Coastal | Cemeteries | Marked social differentiation in burials, including some that are highly elaborate | (Higham 1989, 2004) | ||
| 20 | Oceania | New Guinean fisher-foragers (e.g., Asmat) | 1960 AD | Sago, aquatic resources | Coastal | Observed | Villages exceeding 1,000 or 2,000 individuals | Polygynous chiefs; institutionalized status competition | (Knauft 1993; Roscoe 2002, 2006) | |
| 21 | Oceania | Murray River, Australia (e.g., Yaraldi) | 1860 AD | Broad-spectrum (freshwater, marine, and terrestrial resources) | Riparian and lacustrine | Observed | Clans politically consolidated | Hierarchy of authority, with clan and paramount headmen | Managed tubers to ensure productivity | (Berndt et al. 1993) |
| 22 | Oceania | Southwest Victoria, Australia | 2,000 BP-1850 AD | Aquatic wetland resources (esp. eel) & terrestrial plants (e.g., tubers, ferns) | Coastal plain | Observed | Tribe (composed of many bands)had common dialect and was primary political unit | Observed: Societies dominated by prestigious, powerful, polygynous patriarchal figures; evidence of hereditary transfer | Large-scale artificial drainage systems used in connection with fishing, esp. eeling | (Lourandos 1980, 1983) |
| 23 | North America | Thule | 1100-1500 AD | Bowhead whale, as well as caribou, fish, seals, and bears | Coastal (warm period) | Semi-sedentary seasonal communities with heavy whalebone houses | Villages of 15-25 dwellings normal; one village with >60 | Indications of within-village hierarchies, with headman-leaders | (Savelle 2002; Whitridge 1999) | |
| 24 | North America | Pacific Northwest Indians (e.g., Tlingit, Haida) | 3,500 BP-1900 AD | Terrestrial and aquatic resources, especially anadromous fish | Coastal | Observed; archaeol: substantial houses; villages | Large villages observed | Observed (chiefs, potlatches, slavery); archaeol: burial patterns, cranial deformation, grave goods | Clam gardens; gardening techniques (e.g., soil improvement, plant relocation) | (Ames 1994; Smith 2011) |
| 25 | North America | Interior Plateau, British Columbia | 2,000-1,000 BP | Terrestrial and aquatic resources, especially anadromous fish | Canyon/river drainage | Villages with pit houses | Sites with up to 120 pit houses; large houses | Lavish grave goods, including with children | (Hayden et al. 1985; Hayden and Ryder 1991) | |
| 26 | North America | Chumash & ancestors | 6,500 BP-1770 AD | Marine resources, trade with mainland | Coastal islands | Observed; archaeol: permanent houses (storage pits, hearths, whale bone structure) | Observed | Observed; socially differentiated burials (starting c. 1300 AD) | Translocation of foxes from mainland to islands | (Arnold 1992; Rick et al. 2005) |
| ID | Region | Culture/Subregion | Time | Subsistence | Environment | Reduced mobility | Large groups | Inequality1 | Resource management | References |
| 27 | North America | St. George River Drainage, Maine | 5,000 BP–1650 AD | Shellfish, fish (e.g., cod, swordfish), deer, birds | Coastal | Multi-season occupation, structure building, large middens surrounded by smaller middens | . | . | . | (Eldridge 1990) |
| 28 | North America | Libben | 800–1100 AD | Riparian resources (incl. fish, small mammals, migratory birds)2 | Riparian | Cemetery; homogenous, stable occupation | . | . | . | (Meindl et al. 2008) |
| 29 | North America | Indian Knoll | 6,100–4,500 BP | Aquatic and terrestrial resources (e.g., shellfish, deer) | Riparian | Middens; large cemeteries | . | . | . | (Rothschild 1979; Webb 1974) |
| 30 | North America | Calusa | 800–1550 AD | Marine resources and C3 plants (e.g., tree fruits, tubers)3 | Coastal | Observed | 50 unified villages; domain covered southern FL | Hereditary sovereign for life (“king”) | “Watercourts” (possibly for fish capture and storage) | (Marquardt 2014; Thompson et al. 2018, 2020) |
| 31 | South America | Chinchorro | 7,000–4,000 BP | Marine resources (e.g., fish, sea lions, shellfish); some plants and terrestrial meat | Coastal | Middens; dense mortuary sites; resource distribution | Large shell middens; dense mortuary sites | Differentiation in mummification | . | (Aufderheide et al. 1993; Marquet et al. 2012) |
| 32 | South America | Puna (high altitude Andean grasslands) | 6,200–3,500 BP | Camelids | Arid high plateau | Stone-made habitation structures; storage pits | . | Elaborate burials | Camelid penning | (Yacobaccio 2004; Yacobaccio et al. 2017) |
| 33 | South America | Southeastern coastal Brazil | 4,000–2,000 BP | Marine and some terrestrial resources (e.g., fish, shellfish, tapir, whale, dolphin) | Coastal | Monumental middens; specialized burial areas used for millennia | 1,575 people buried per 25 years in places (44,000 over 700 y) | Substantial differentiation in burials, including elaborate grave goods | . | (de Blasis et al. 1998; Fish and Fish 2010) |
| 34 | South America | Plata-Purana Wetlands | 1,700 BP–1500 AD | Wetlands resources (e.g., fish, large rodents, deer, palm) | Coastal wetlands | Pottery; burial sites differentiated from habitation sites | . | .4 | . | (Loponte et al. 2006) |
1 Inequality includes substantial differences in material wealth, institutionalized status hierarchies (e.g., class differentiation with privileges), and/or institutionalized political authority (e.g., chiefs).
2 There is evidence of maize cultivation at some point during the occupation.
3 Although recent evidence suggests the Calusa cultivated squash and papaya, in addition to managing chile pepper (Marquardt 2014), archaeological and isotopic analyses indicate these represented trivial contributions to subsistence (Hutchinson et al. 2016).
4 Loponte et al. (2006) include the Chaná-Timbú and other wetland groups observed by early Spanish chroniclers in the “hunter-gatherer complex” of the Plata-Purana wetlands. These peoples stored fish, owned fishing locations, had substantial dwellings, and wore labrets. They exhibited low mobility, year-round permanence in the wetlands, and a formalized chief system. However, their foraging economy was supplemented, possibly substantially, by the cultivation of domesticates, particularly maize. Although maize may have been adopted in this sixteenth century—early observers referred to them exclusively as foragers—we exclude these groups here, instead relying on archaeologically documented foragers.
References
Ames, K. M. (1994). The Northwest Coast: Complex hunter-gatherers, ecology, and social evolution. Annual Review of Anthropology 23:209–229. doi:10.1002/evan.10102
Angel, J. L., Phenice, L. H., Robbins, L. H., & Lynch, B. M. (1980). Late Stone-Age fishermen of Lothagam, Kenya. Michigan State University Museum Anthropological Series 3:143–201.
Arnold, J. E. (1992). Complex hunter-gatherer-fishers of prehistoric California: Chiefs, specialists, and maritime adaptations of the Channel Islands. American Antiquity 57:60–84.
Aufderheide, A. C., Muñoz, I., & Arriaza, B. (1993). Seven Chinchorro mummies and the prehistory of northern Chile. American Journal of Physical Anthropology 91:189–201. doi:10.1002/ajpa.1330910205
Bar-Yosef, O. (1994). The Natufian culture in the Levant, threshold to the origins of agriculture. Evolutionary Anthropology 21:159–177. doi:10.2307/530343
Barton, R. N. E., Bouzouggar, A., Collcutt, S. N., & Humphries, L. T. (2019). Cemeteries and sedentism in the later Stone Age of NW Africa: Excavations at Grotte des Pigeons, Taforalt, Morocco. Römiisch Germanisches Zentralmuseum.
Belfer-Cohen, A., & Bar-Yosef, O. (2000). Early sedentism in the Near East: A bumpy ride to village life.
In I. Kuijt (Ed.), Life in Neolithic farming: Social organization, identity, and differentiation (pp. 19–37). Kluwer Academic/Plenum Publishers. doi:10.1016/s0140-6736(01)79764-4
Berndt, R. M., Berndt, C. H., & Stanton, J. E. (1993). A world that was: The Yaraldi of the Murray River and the lakes , south Australia. University of British Columbia Press.
Chi, Z., & Hung, H. (2012). Later hunter-gatherers in southern China, 18000 – 3000 BC. Antiquity 86:11–29.
de Blasis, P., Fish, S. K., Gaspar, M. D., & Fish, P. R. (1998). Some references for the discussion of complexity among the sambaqui moundbuilders from the southern shores of Brazil. Revista de Arquelogía Americana 75–105. doi:10.2307/27768414
Eldridge, S. A. (1990). Ceramic period occupation of the central Maine coast: Hunter-gatherer estuarine adaptations in the St. George River drainage. University of Pennsylvania.
Fish, S. K., & Fish, P. R. (2010). Monumentality and complex hunter-gatherers in southeast coastal Brazil. In D. Calado, M. Baldia, & M. Boulanger (Eds.), Monumental Questions: Prehistoric Megaliths, Mounds and Enclosures (pp. 231–236). British Archaeological Reports.
Garcea, E. A. A. (2006). Semi-permanent foragers in semi-arid environments of North Africa. World Archaeology 38:197–219. doi:10.1080/00438240600693968
Habu, J. (2004). Ancient Jomon of Japan. Cambridge University Press.
Hall, S., & Binneman, J. (1987). Later stone age burial variability in the Cape: A social interpretation. The South African Archaeological Bulletin 42:140–152.
Hayden, B., Eldridge, M., Eldridge, A., & Cannon, A. (1985). Complex hunter-gatherers in interior British Columbia. In D. T. Price & J. A. Brown (Eds.), Prehistoric hunter-gatherers: The emergence of complexity. Academic Press, Inc.
Hayden, B., & Ryder, J. M. (1991). Prehistoric cultural collapse in the Lillooet area. American Antiquity 56:50–65.
Higham, C. F. W. (1989). Social Organisation at Khok Phanom Di, Central Thailand (2000-1500 B.C.). Arts asiatiques 44:25–43. doi:10.3406/arasi.1989.1256
Higham, C. F. W. (2004). Khok Phanom Di, Archaeology of. In (C. Smith, Ed.)Encylopedia of global archaeology. Springer Reference.
Hogue, J. T. (2014). The origin and development of the Pleistocene LSA in Northwest Africa: A case study from Grotte des Pigeons (Taforalt), Morocco. University of Oxford.
Hutchinson, D. L., Norr, L., Schober, T., Marquardt, W. H., Walker, K. J., Newsom, L. A., & Scarry, C. M. (2016). The Calusa and prehistoric subsistence in central and south Gulf Coast Florida. Journal of Anthropological Archaeology 41:55–73. doi:10.1016/j.jaa.2015.10.004
Jerardino, A. (2012). Large Shell Middens and Hunter-Gatherer Resource Intensification Along the West Coast of South Africa: The Elands Bay Case Study. Journal of Island and Coastal Archaeology 7:76–101. doi:10.1080/15564894.2010.541551
Jerardino, A., & Navarro, R. (2018). Large-scale hunter-gatherer exploitation of marine resources in South Africa, Part II: Grootrif and malkoppan megamiddens, lamberts bay area. South African Archaeological Bulletin 73:108–125.
Knauft, B. M. (1993). South coast New Guinea cultures: History, comparison, dialectic. Cambridge University Press. doi:10.16309/j.cnki.issn.1007-1776.2003.03.004
Kriiska, A., Oras, E., Lõugas, L., Meadows, J., Lucquin, A., & Craig, O. E. (2017). Late Mesolithic Narva
Stage in Estonia: Pottery, Settlement Types and Chronology. Estonian Journal of Archaeology 21:52. doi:10.3176/arch.2017.1.03
Loponte, D., Acosta, A., & Musali, J. (2006). Complexity among hunter-gatherers from the Pampean region, South America. In C. Grier, J. Kim, & J. Uchiyama (Eds.), Beyond affluent foragers: Rethinking hunter-gatherer complexity (pp. 106–125). Oxbow Books. doi:10.2307/j.ctt1w0df3b.12
Lourandos, H. (1980). Change or stability?: Hydraulics, hunter-gatherers and population in temperate Australia. World Archaeology 11:245–264. doi:10.1080/00438243.1980.9979765
Lourandos, H. (1983). Intensification: A late Pleistocene-Holocene archaeological sequence from southwestern Victoria. Archaeology in Oceania 18:81–94.
Maritan, L., Iacumin, P., Zerboni, A., Venturelli, G., Dal Sasso, G., Linseele, V., et al. (2018). Fish and salt: The successful recipe of White Nile Mesolithic hunter-gatherer-fishers. Journal of Archaeological Science 92:48–62. doi:10.1016/j.jas.2018.02.008
Marquardt, W. H. (2014). Tracking the Calusa: A retrospective. Southeastern Archaeology 33:1–24. doi:10.1179/sea.2014.33.1.001
Marquet, P. A., Santoro, C. M., Latorre, C., Standen, V. G., Abades, S. R., Rivadeneira, M. M., et al. (2012). Emergence of social complexity among coastal hunter-gatherers in the Atacama Desert of northern Chile. Proceedings of the National Academy of Sciences 109:14754–14760. doi:10.1073/pnas.1116724109
Meindl, R. S., Mensforth, R. P., & Lovejoy, C. O. (2008). The Libben Site: A hunting, fishing, and gathering village from the eastern late woodlands of North America. Analysis and implications for palaeodemography and human origins. Recent advances in palaeodemography: Data, techniques, patterns. doi:10.1007/978-1-4020-6424-1_9
Nasu, H., & Momohara, A. (2016). The beginnings of rice and millet agriculture in prehistoric Japan. Quaternary International 397:504–512. doi:10.1016/j.quaint.2015.06.043
Oras, E., Lucquin, A., Lõugas, L., Tõrv, M., Kriiska, A., & Craig, O. E. (2017). The adoption of pottery by north-east European hunter-gatherers: Evidence from lipid residue analysis. Journal of Archaeological Science 78:112–119. doi:10.1016/j.jas.2016.11.010
Prendergast, M. E. (2010). Kansyore fisher-foragers and transitions to food production in East Africa: The view from Wadh Lang’o, Nyanza Province, Western Kenya. Azania 45:83–111. doi:10.1080/00672700903291765
Price, M., & Hongo, H. (2019). The archaeology of pig domestication in Eurasia. Journal of Archaeological Research. doi:10.1007/s10814-019-09142-9
Price, T. D. (2015). Ancient Scandinavia: An archaeological history from the first humans to the Vikings. Oxford University Press.
Price, T. D., & Bar-Yosef, O. (2010). Traces of inequality at the origins of agriculture in the ancient Near East. In T. D. Price & G. M. Feinman (Eds.), Pathways to power (pp. 147–168). Springer. doi:10.1007/978-1-4419-6300-0_6
Rick, T. C., Erlandson, J. M., Vellanoweth, R. L., & Braje, T. J. (2005). From Pleistocene mariners to complex hunter-gatherers: The archaeology of the California Channel Islands. Journal of World Prehistory 19:169–228. doi:10.1007/s10963-006-9004-x
Roscoe, P. (2002). The hunters and gatherers of New Guinea. Current Anthropology 43:153–162. doi:10.1086/338289
Roscoe, P. (2006). Fish, game, and the foundations of complexity in forager society: The evidence from New Guinea. Cross-Cultural Research 40:29–46. doi:10.1177/1069397105282432
Rössner, C., Deckers, K., Benz, M., Özkaya, V., & Riehl, S. (2018). Subsistence strategies and vegetation development at Aceramic Neolithic Körtik Tepe, southeastern Anatolia, Turkey.
Vegetation History and Archaeobotany 27:15–29. doi:10.1007/s00334-017-0641-z
Rothschild, N. A. (1979). Mortuary behavior and social organization at Indian Knoll and Dickson Mounds. American Antiquity 44:658–675.
Rowley-Conwy, P. (1999). Economic Prehistory in Southern Scandinavia. Proceedings of the British Academy 125–160.
Salvatori, S. (2012). Disclosing Archaeological Complexity of the Khartoum Mesolithic: New Data at the
Site and Regional Level. African Archaeological Review 29:399–472. doi:10.1007/s10437-012-9119-7
Salvatori, S., & Usai, D. (2019). The Mesolithic and Neolithic in Sudan. In D. Raue (Ed.), Handbook of ancient Nubia, vol. 1 (pp. 171–193). de Gruyter.
Savelle, J. M. (2002). Logistical organization, social complexity, and the collapse of prehistoric Thule whaling societies in the central Canadian Arctic archipelago. In B. Fitzhugh & J. Habu (Eds.), Beyond foraging and collecting: Evolutionary change in hunter-gatherer settlement systems (pp. 73–90). Kluwer Academic/Plenum Publishers. doi:10.1007/978-1-4615-0543-3
Sealy, J. (2006). Diet, mobility, and settlement pattern among Holocene hunter-gatherers in southernmost Africa. Current Anthropology 47:569–595. doi:10.1086/504163
Sereno, P. C., Garcea, E. A. A., Jousse, H., Stojanowski, C. M., Saliège, J. F., Maga, A., et al. (2008).
Lakeside cemeteries in the Sahara: 5000 years of holocene population and environmental change. PLoS ONE 3. doi:10.1371/journal.pone.0002995
Smith, B. D. (2011). General patterns of niche construction and the management of “wild” plant and animal resources by small-scale pre-industrial societies. Philosophical Transactions of the Royal
Society B: Biological Sciences 366:836–848. doi:10.1098/rstb.2010.0253
Soffer, O. (1985). Patterns of Intensification as Seen from the Upper Paleolithic of the Central Russian Plain. In D. T. Price & J. A. Brown (Eds.), Prehistoric hunter-gatherers: The emergence of complexity (pp. 232–270). Academic Press, Inc.
Soffer, O. (1989). Storage, sedentism and the Eurasian Palaeolithic record. Antiquity 63:719–732.
Stojanowski, C. M., & Knudson, K. J. (2011). Biogeochemical inferences of mobility of early Holocene fisher-foragers from the Southern Sahara Desert. American Journal of Physical Anthropology 146:49–61. doi:10.1002/ajpa.21542
Svoboda, J. A. (2016). Dolní Věstonice – Pavlov. Regionální muzeum v Mikulově.
Tallavaara, M., & Pesonen, P. (2020). Human ecodynamics in the north-west coast of Finland 10,000–2000 years ago. Quaternary International 549:26–35. doi:10.1016/j.quaint.2018.06.032
Thompson, V. D., Marquardt, W. H., Savarese, M., Walker, K. J., Newsom, L. A., Lulewicz, I., et al. (2020). Ancient engineering of fish capture and storage in southwest Florida. Proceedings of the National Academy of Sciences of the United States of America 117:8374–8381. doi:10.1073/pnas.1921708117
Thompson, V. D., Marquardt, W. H., Walker, K. J., Thompson, A. D. R., & Newsom, L. A. (2018). Collective action, state building, and the rise of the Calusa, southwest Florida, USA. Journal of Anthropological Archaeology 51:28–44. doi:10.1016/j.jaa.2018.05.003
Trinkaus, E. (2005). The adiposity paradox in the middle danubian Gravettian. Anthropologie (Brno) 43:263–271.
Trinkaus, E., & Svoboda, J. A. (Eds.). (2006). Early modern human evolution in Central Europe: The people of Dolní Věstonice and Pavlov. Oxford University Press.
Vaneeckhout, S. (2010). House societies among coastal hunter-gatherers: A case study of stone age Ostrobothnia, Finland. Norwegian Archaeological Review 43:12–25. doi:10.1080/00293652.2010.499003
Viet, N. (2005). The Da But culture: Evidence for cultural development in Vietnam during the Middle Holocene. Bulletin of the Indo-Pacific Prehistory Association 25:89–93. doi:10.7152/bippa.v25i0.11918
Webb, W. (1974). Indian Knoll. The University of Tennessee Press.
Whitridge, P. J. (1999). The construction of social difference in a prehistoric Inuit whaling community. Arizona State University.
Xianguo, F. (2002). The Dingsishan site and the prehistory of Guangxi, South China. Bulletin of the IndoPacific Prehistory Association 22:63–71.
Yacobaccio, H. D. (2004). Social dimensions of camelid domestication in the Southern Andes. Anthropozoologica 39:237–247.
Yacobaccio, H. D., Morales, M. R., & Hoguin, R. (2017). Habitats of ancient hunter-gatherers in the Puna:
Resilience and discontinuities during the Holocene. Journal of Anthropological Archaeology 46:92–100. doi:10.1016/j.jaa.2016.08.004